Abstract
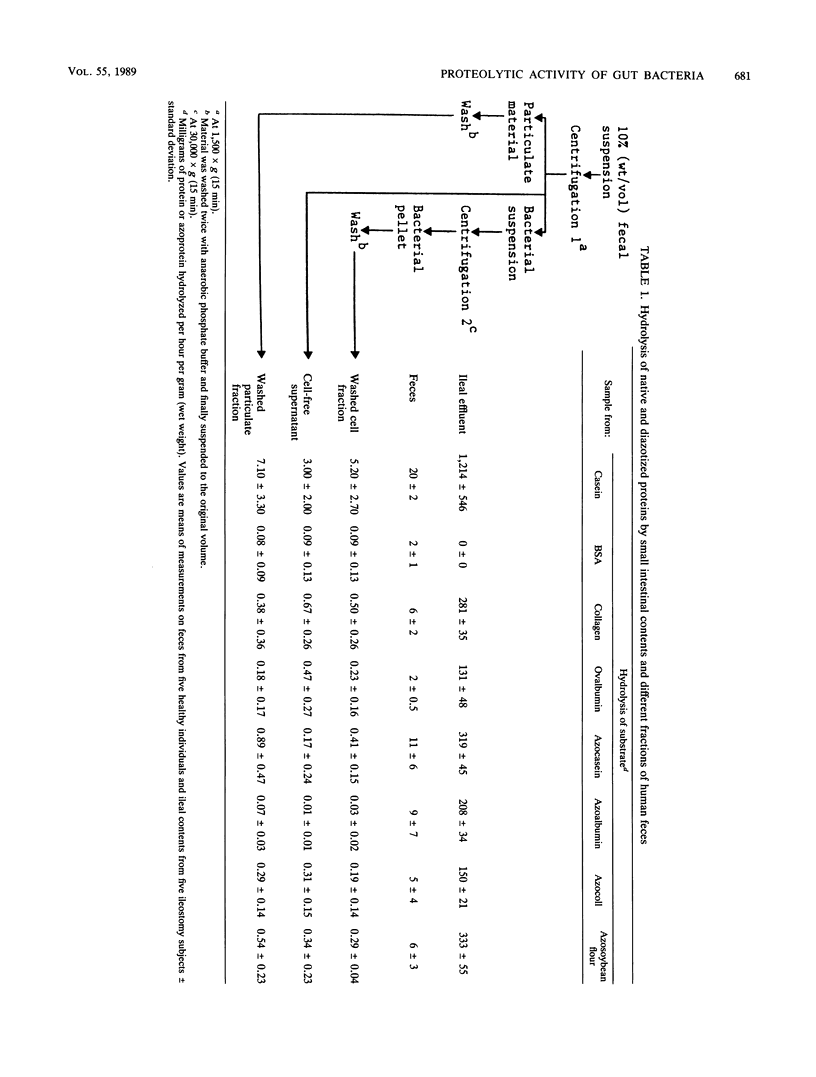
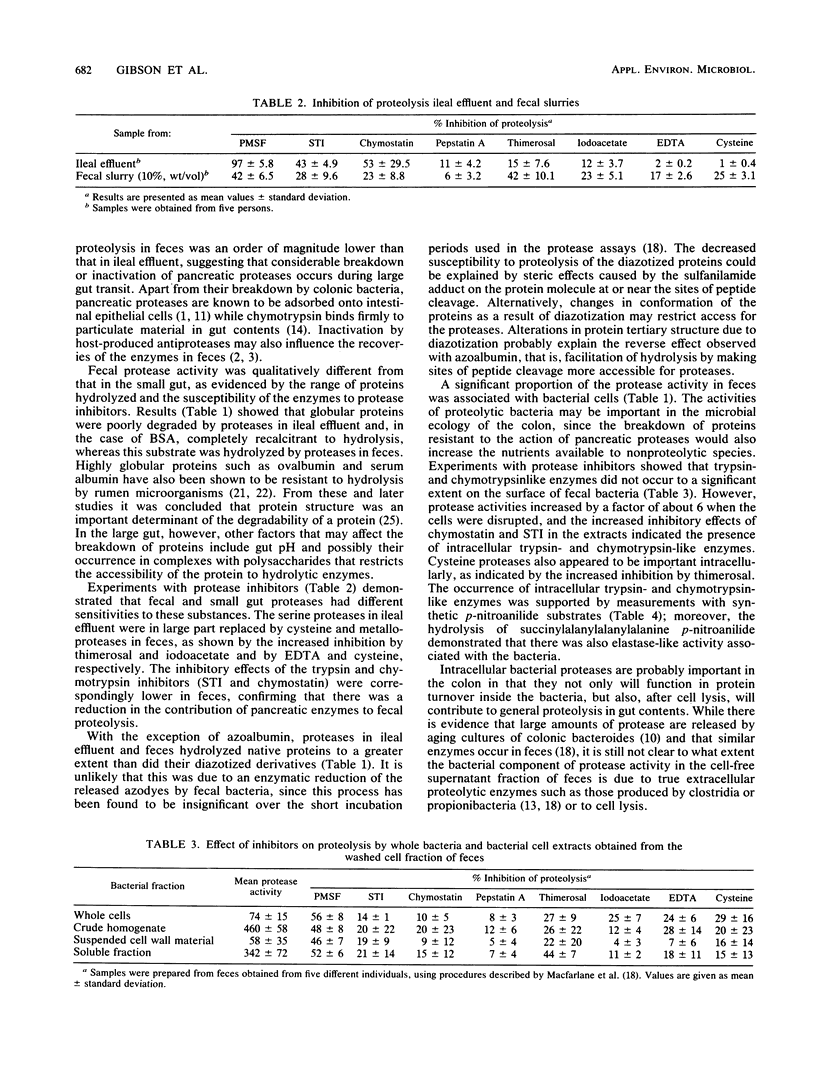
Protease activities in human ileal effluent and feces were compared by using a variety of native and diazotized protein substrates. In many cases the diazotized proteins had altered susceptibilities to hydrolysis compared with the native proteins. Proteolytic activity was significantly greater than (P less than 0.001) in small intestinal effluent than in feces (319 +/- 45 and 11 +/- 6 mg of azocasein hydrolyzed per h per g, respectively). Moreover, fecal proteolysis was qualitatively different in that ileal effluent did not hydrolyze the highly globular protein bovine serum albumin, whereas all fecal samples tested degraded this substrate. Inhibition experiments provided further evidence that fecal protease activity differed from that in the small intestine. Physical disruption of fecal bacteria released large quantities of proteases, indicating that the lysis of bacteria in the colon may contribute to the extracellular proteolytic activity in feces. Protease inhibition studies with washed fecal bacteria showed that they produced serine, cystine, and metalloproteases, and experiments with synthetic p-nitroanilide substrates indicated that low levels of trypsin- and chymotrypsin-like activities were associated with whole cells. An elastase-like enzyme was bound to the outer membranes of some fecal bacteria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohe M., Borgström A., Genell S., Ohlsson K. Determination of immunoreactive trypsin, pancreatic elastase and chymotrypsin in extracts of human feces and ileostomy drainage. Digestion. 1983;27(1):8–15. doi: 10.1159/000198913. [DOI] [PubMed] [Google Scholar]
- Bohe M., Genell S., Ohlsson K. Protease inhibitors in plasma and faecal extracts from patients with active inflammatory bowel disease. Scand J Gastroenterol. 1986 Jun;21(5):598–604. doi: 10.3109/00365528609003106. [DOI] [PubMed] [Google Scholar]
- Catassi C., Cardinali E., D'Angelo G., Coppa G. V., Giorgi P. L. Reliability of random fecal alpha 1-antitrypsin determination on nondried stools. J Pediatr. 1986 Sep;109(3):500–502. doi: 10.1016/s0022-3476(86)80128-7. [DOI] [PubMed] [Google Scholar]
- Chacko A., Cummings J. H. Nitrogen losses from the human small bowel: obligatory losses and the effect of physical form of food. Gut. 1988 Jun;29(6):809–815. doi: 10.1136/gut.29.6.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H. Cellulose and the human gut. Gut. 1984 Aug;25(8):805–810. doi: 10.1136/gut.25.8.805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H., Hill M. J., Bone E. S., Branch W. J., Jenkins D. J. The effect of meat protein and dietary fiber on colonic function and metabolism. II. Bacterial metabolites in feces and urine. Am J Clin Nutr. 1979 Oct;32(10):2094–2101. doi: 10.1093/ajcn/32.10.2094. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Pomare E. W., Branch W. J., Naylor C. P., Macfarlane G. T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987 Oct;28(10):1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings J. H. Short chain fatty acids in the human colon. Gut. 1981 Sep;22(9):763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson S. A., Macfarlane G. T. Studies on the proteolytic activity of Bacteroides fragilis. J Gen Microbiol. 1988 Jan;134(1):19–27. doi: 10.1099/00221287-134-1-19. [DOI] [PubMed] [Google Scholar]
- Goldberg D. M., Campbell R., Roy A. D. Binding of trypsin and chymotrypsin by human intestinal mucosa. Biochim Biophys Acta. 1968 Nov 19;167(3):613–615. doi: 10.1016/0005-2744(68)90053-3. [DOI] [PubMed] [Google Scholar]
- Ingram E., Holland K. T., Gowland G., Cunliffe W. J. Studies of the extracellular proteolytic activity produced by Propionibacterium acnes. J Appl Bacteriol. 1983 Apr;54(2):263–271. doi: 10.1111/j.1365-2672.1983.tb02616.x. [DOI] [PubMed] [Google Scholar]
- KUKRAL J. C., ADAMS A. P., PRESTON F. W. PROTEIN PRODUCING CAPACITY OF THE HUMAN EXOCRINE PANCREAS: INCORPORATION OF S35 METHIONINE IN SERUM AND PANCREATIC JUICE PROTEIN. Ann Surg. 1965 Jul;162:63–73. doi: 10.1097/00000658-196507000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaspar P., Möller G., Wahlefeld A. New photometric assay for chymotrypsin in stool. Clin Chem. 1984 Nov;30(11):1753–1757. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Macfarlane G. T., Allison C., Gibson S. A., Cummings J. H. Contribution of the microflora to proteolysis in the human large intestine. J Appl Bacteriol. 1988 Jan;64(1):37–46. doi: 10.1111/j.1365-2672.1988.tb02427.x. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Cummings J. H., Allison C. Protein degradation by human intestinal bacteria. J Gen Microbiol. 1986 Jun;132(6):1647–1656. doi: 10.1099/00221287-132-6-1647. [DOI] [PubMed] [Google Scholar]
- Macfarlane G. T., Englyst H. N. Starch utilization by the human large intestinal microflora. J Appl Bacteriol. 1986 Mar;60(3):195–201. doi: 10.1111/j.1365-2672.1986.tb01073.x. [DOI] [PubMed] [Google Scholar]
- Mahadevan S., Erfle J. D., Sauer F. D. Degradation of soluble and insoluble proteins by Bacteroides amylophilus protease and by rumen microorganisms. J Anim Sci. 1980 Apr;50(4):723–728. doi: 10.2527/jas1980.504723x. [DOI] [PubMed] [Google Scholar]
- Mangan J. L. Quantitative studies on nitrogen metabolism in the bovine rumen. The rate of proteolysis of casein and ovalbumin and the release and metabolism of free amino acids. Br J Nutr. 1972 Mar;27(2):261–283. doi: 10.1079/bjn19720092. [DOI] [PubMed] [Google Scholar]
- McNeil N. I., Cummings J. H., James W. P. Short chain fatty acid absorption by the human large intestine. Gut. 1978 Sep;19(9):819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas T. D., Jarvis B. D., Skipper N. A. Localization of proteinase(s) near the cell surface of Streptococcus lactis. J Bacteriol. 1974 May;118(2):329–333. doi: 10.1128/jb.118.2.329-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J. Adsorption of soluble proteins to rumen bacteria and the role of adsorption in proteolysis. Br J Nutr. 1985 Mar;53(2):399–408. doi: 10.1079/bjn19850047. [DOI] [PubMed] [Google Scholar]


