Abstract
Epileptic seizures are associated with increases in hippocampal excitability, but the mechanisms that render the hippocampus hyperexcitable chronically (in epilepsy) or acutely (in status epilepticus) are poorly understood. Recent evidence suggests that substance P (SP), a peptide that has been implicated in cardiovascular function, inflammatory responses, and nociception, also contributes to hippocampal excitability and status epilepticus, in part by enhancing glutamate release. Here we report that mice with disruption of the preprotachykinin A gene, which encodes SP and neurokinin A, are resistant to kainate excitoxicity. The mice show a reduction in the duration and severity of seizures induced by kainate or pentylenetetrazole, and both necrosis and apoptosis of hippocampal neurons are prevented. Although kainate induced the expression of bax and caspase 3 in the hippocampus of wild-type mice, these critical intracellular mediators of cell death pathways were not altered by kainate injection in the mutant mice. These results indicate that the reduction of seizure activity and the neuroprotection observed in preprotachykinin A null mice are caused by the extinction of a SP/neurokinin A-mediated signaling pathway that is activated by seizures. They suggest that these neurokinins are critical to the control of hippocampal excitability, hippocampal seizures, and hippocampal vulnerability.
Status epilepticus (SE), a state of severe, repetitive seizure activity, still caries a 27% mortality and frequently leads to widespread neuronal death (1). The mechanisms of self-sustaining SE and SE-induced brain damage are unknown, but may in part be excitotoxic, i.e., resulting from excessive stimulation of neurons by the excitatory amino acid glutamate (2–4). Consistent with the evidence that substance P (SP) and other tachykinins modulate glutamatergic excitatory synaptic transmission via AMPA, NMDA, and kainate receptors (5–8), we recently reported that intrahippocampal injection of picomolar amounts of SP can trigger SE, generating a pattern of hippocampal damage that resembles that which occurs in human epilepsy. We also showed that SP receptor antagonists block both the development and maintenance of self-sustaining SE (9). These data, together with evidence that SP receptor antagonists reduce kainic acid-induced seizure activity (10) and ischemic injury (11) in rat brain, suggest that tachykinins are essential in generating limbic seizures and in hippocampal-selective neuronal vulnerability. To test this hypothesis, we have used a genetic approach and found that preprotachykinin A (PPT-A)-deficient (PPT-A−/−) mice (12) exhibit a remarkable reduction in kainate-induced seizures and neuronal death.
Materials and Methods
Induction and Analysis of Seizures.
Wild-type (PPT-A+/+) and PPT-A(−/−) mice were injected i.p. with kainate (Sigma) at 25 or 35 mg/kg or pentylenetetrazole (PTZ, Sigma) at 50 mg/kg. Seizure activity was videotaped during an observation period of 3 h (for kainate) or 30 min (for PTZ). The times of seizure onset and seizure duration were recorded. Behavioral seizures were scored according to a previously defined scale (13): stage 1, exploring, sniffing, and grooming ceased and the mice became motionless; stage 2, forelimb and/or tail extension, giving the appearance of a rigid posture; stage 3, myoclonic jerks of the head and neck, with brief twitching movements; stage 4, forelimb clonus and partial rearing; stage 5, forelimb clonus, rearing, and falling; and stage 6, generalized tonic-clonic activity with loss of postural tone, often resulting in death.
Kainate-Induced Hippocampal Damage.
PPT-A(+/+) and PPT-A(−/−) mice were perfused with 4% paraformaldehyde (for light microscopy) or 3% glutaraldehyde (for electron microscopy, EM) 3 or 7 days after the injection of kainate (35 mg/kg). Coronal frozen sections (30 μm thick) through hippocampi were stained with hematoxylin/eosin to assess acute neuronal damage or with cresyl violet to determine chronic neuronal loss. We used a stereological approach to count neurons in the pyramidal cell layers (14). EM of hippocampus was used to better assess and quantify features of apoptotic and/or necrotic death. Seventy-micrometer-thick vibratome sections through the hippocampus were processed as described (15). Ultrathin sections from CA1, CA3, and the dentate hilus were examined by transmission EM. We counted the total number of apoptotic and necrotic neurons in six ultrathin sections on single slot grids per block, with three blocks minimum for each animal. Neurons were considered to be apoptotic when cytoplasmic organelles and plasma membrane were relatively preserved, and chromatin was condensed into a few round clumps (16). Neurons were counted as necrotic when they displayed swelling, cytolysis with membrane breaks, and a pyknotic nucleus with irregular contour of the chromatin.
Terminal Transferase-Mediated Biotinylated-UTP Nick End-Labeling (TUNEL).
PPT-A(−/−) and control PPT-A(+/+) mice were perfused with 4% paraformaldehyde 3 days after the injection of kainate (35 mg/kg). Coronal frozen sections (30 μm thick) through the hippocampi were stained by in situ end-labeling of nuclear DNA fragments (17) using terminal deoxynucleotidyl transferase and biotinylated dUTP (Trivigen, Gaithersburg, MD) as substrate. Incorporated biotin was detected by using ABC (Vector Laboratories) and 3,3′-diaminobenzidine as chromogen. Quantification of nuclear DNA fragmentation was carried out in CA1 and CA3.
Kainate Induction of Cell Death Genes.
PPT-A(+/+) and PPT-A(−/−) mice were perfused with 4% paraformaldehyde 3 days after the induction of SE with kainate (35 mg/kg). Coronal frozen sections (30 μm thick) through the hippocampi were incubated with a rabbit anti-bax antiserum (Oncogene) or a rabbit anti-caspase 3 antiserum (Santa Cruz Biotechnology). The antisera were detected by using ABC (Vector Laboratories) and 3,3-diaminobenzidine (Sigma) with nickel enhancement. The specificity of antisera was verified by omitting the primary antisera, in which case there was no labeling. We used a stereological approach to quantify the immunoreactivity (14). The total number of immunoreactive neurons in every fourth section in the CA1 and CA3 subfields was counted from video monitor images of the sections.
Results
PPT-A(−/−) mice are viable, fertile, and of normal size (12). They have an outwardly normal behavior and activity, and normal levels of the three major neurokinin (NK) receptors (NK-1, -2, and -3) in the central nervous system and peripheral tissues (12). Histological surveys of a variety of brain tissues revealed no apparent abnormality (data not shown). However, these mice exhibit defects in the response to intense noxious stimuli (12).
PPT-A(−/−) Mice Are Resistant to Chemically Induced Seizures.
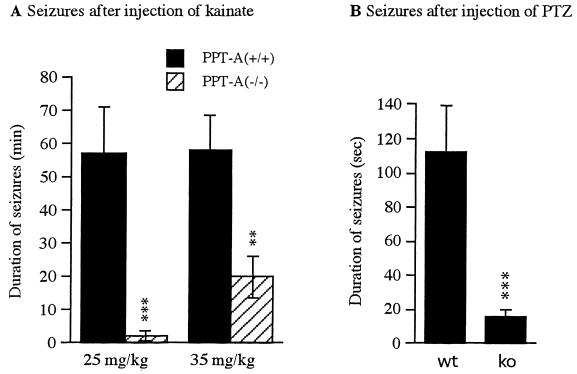
The phenotype of PPT-A deficiency became apparent when mice were injected i.p. with an epileptogenic dose of kainate, which elicits seizures directly by stimulation of glutamate receptors, and indirectly by increasing the release of excitatory amino acids from nerve terminals (18). Within a few minutes after kainate injection, PPT-A(+/+) mice first became motionless, progressed to rigid posture with forelimb and tail extension, exhibited repetitive scratching with “wet-dog shakes,” rearing and falling, and eventually developed nearly continuous severe, tonic-clonic seizures (Table 1). A majority of animals exhibited strong to severe seizures during the first 20–60 min after injection. In contrast, PPT-A(−/−) mice showed seizures of much shorter duration (Fig. 1A) and lower intensity (Table 1), mainly consisting of freezing and occasional clonic activity. The onset of seizures was delayed [25 ± 9 min in PPT-A(−/−) vs. 17 ± 7 min in PPT-A(+/+) mice], and recovery was more rapid [58 ± 13 min in PPT-A(−/−) vs. 83 ± 16 min in PPT-A(+/+) mice]. PPT-A(−/−) mice were capable of severe seizures, as shown by treatment with a higher dosage of kainate (5/10 had stage 4 seizures at 35 mg/kg). However, at such high dosage, more than 70% of PPT-A(+/+) mice displayed stage 6 seizure, and 30% died from continuous tonic-clonic convulsions. Stage 6 seizure and death occurred in 10% of PPT-A(−/−) mice (Table 1). We also examined the susceptibility of those mice to PTZ, another epileptogenic agent. PTZ (50 mg/kg) i.p. caused an average of 112 ± 26 sec of clonic or tonic seizures (Fig. 1B, Table 1) in PPT-A(+/+) mice, but much milder and shorter seizures (16 ± 3 sec, P < 0.003) in the PPT-A(−/−) mice.
Table 1.
Classification of seizure scores in PPT-A(+/+) and PPT-A(−/−) mice after systemic administration of kainate and PTZ
| Mouse type | Dose mg | Seizure scores, % of mice
|
Mortality | |||||
|---|---|---|---|---|---|---|---|---|
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | Stage 5 | Stage 6 | |||
| WT (n = 6) | 25 (KA) | 6 (100) | 6 (100) | 6 (100) | 4 (66) | 3 (50) | 1 (17) | 0 (0) |
| KO (n = 6) | 25 (KA) | 6 (100) | 6 (100) | 4 (66) | 1 (16)a | 0 (0)c | 0 (0) | 0 (0) |
| WT (n = 20) | 35 (KA) | 20 (100) | 20 (100) | 18 (90) | 16 (80) | 14 (70) | 10 (50) | 6 (30) |
| KO (n = 10) | 35 (KA) | 10 (100) | 10 (100) | 8 (80) | 5 (50)a | 1 (12)c | 1 (10)b | 1 (10)a |
| WT (n = 12) | 50 (PTZ) | 12 (100) | 12 (100) | 12 (100) | 8 (66) | 7 (58) | ||
| KO (n = 10) | 50 (PTZ) | 10 (100) | 10 (100) | 8 (80) | 3 (30)a | 1 (10)c | ||
WT, PPT-A(+/+); KO, PPT-A(−/−). Comparison of seizure responses between WT and KO mice. KA, kainate. a, P < 0.05; b, P < 0.01; c, P < 0.001.
Figure 1.
Comparison of seizure duration in littermates of varied PPT-A genotypes. (A) Cumulative duration of seizures in PPT-A(+/+) and PPT-A(−/−) mice after i.p. injection of kainate (25 or 35 mg/kg, as indicated). Seizures were recorded for 3 hr after injection of kainate. Data are mean ± SEM and were analyzed by ANOVA with probable least-squares difference (PLSD) Fisher’s test. ★★, P < 0.01; ★★★, P < 0.001, compared with wild-type PPT-A(+/+) animals. (B) Cumulative duration of seizures of PPT-A(+/+) (wt) and PPT-A(−/−) (ko) mice after i.p. injection of PTZ (50 mg/kg). Seizures were recorded for 30 min after injection of PTZ. Data are mean ± SEM and were analyzed by ANOVA with PLSD Fisher’s test. ★★★, P < 0.01, compared with PPT-A(+/+) mice.
PPT-A(−/−) Mice Are Resistant to Kainate-Induced Neuronal Death.
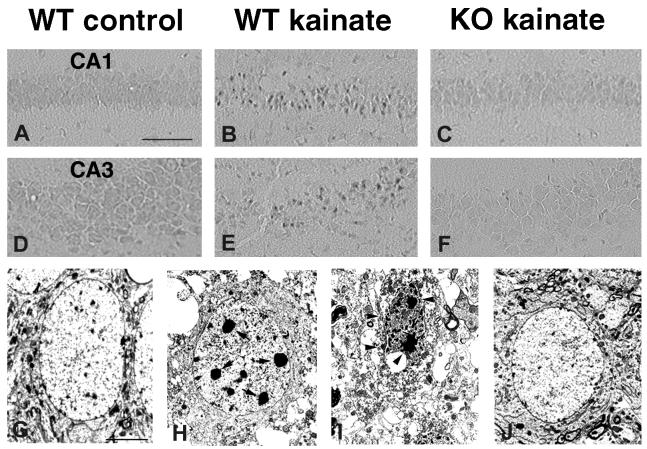
Because SP-triggered SE (9) and other forms of SE (19–21) are associated with neuronal death, and because SP can modulate kainate-induced neuronal death (10) and ischemic neuronal injury (11), we next studied the effects of the null mutation of the PPT-A gene on neuronal survival after kainate SE. We examined 10 PPT-A(+/+) and six PPT-A(−/−) littermates for acute neuronal injury 3 days after systemic administration of 35 mg/kg kainate. Hematoxylin/eosin staining showed many neurons with acidophilic cytoplasm and shrunken, pyknotic nuclei in the hippocampus of PPT-A(+/+) mice (Fig. 2 B and E). This neuronal injury was never seen in uninjected or saline-injected mice (Fig. 2 A and D). Counts of neurons in hematoxylin/eosin-stained tissue (Table 2) showed prominent acute neuronal injury in CA1, CA3, and hilus in seven of 10 wild-type mice. This finding is consistent with CA3 and CA1 being the most kainate-vulnerable regions (22). In contrast, only two of the six PPT-A(−/−) mice displayed evidence of acute neuronal injury of the hippocampus (Table 2), and this injury was mild and restricted to the CA3b subfield (Fig. 2F, Table 2). Because SE is reported to cause delayed excitotoxic neuronal loss (21, 23), we also studied chronic neuronal loss, by using the cresyl violet stain, 7 days after systemic administration of 35 mg/kg kainate. Compared with uninjected mice (Fig. 2G), after kainate treatment we observed neuronal loss, with resultant narrowing and sparse staining of the CA1 region and a breach of continuity of staining in the CA3 region in four of six PPT-A(+/+) mice (Fig. 2H, Table 2). By contrast, we did not detect any cell loss after kainate treatment in the hippocampus of three PPT-A(−/−) mice (Fig. 2I, Table 2).
Figure 2.
Comparison of kainate-induced neuronal damage in the hippocampus of PPT-A(+/+) (WT) and PPT-A(−/−) (KO) mice. The segments shown in both groups are the part of CA1 closest to CA2 and the lower curving portion of CA3 located CA3b. (A-F) Hematoxylin/eosin staining in saline-injected controls (A and D), kainate-treated PPT-A(+/+) (B and E), and kainate-treated PPT-A(−/−) mice (C and F). A large number of injured neurons with shrunken, acidophilic cytoplasm and pyknotic nuclei are seen in CA1 (B) and CA3 (E) pyramidal cell layers of PPT-A(+/+) mice 3 days after injection of kainate (35 mg/kg). In comparable sections of kainate-treated PPT-A(−/−) mice, the acute damage was minimal and confined to CA3b (arrows in F) and was absent from CA1 (C). (G–I) Photomicrographs of cresyl violet-stained hippocampus 7 days after injection of kainate, showing neuronal loss. The sections through dorsal hippocampus of PPT-A(+/+) mice showed a thinned and sparsely staining CA1 pyramidal cell layer (arrows in H) and a breach of staining in CA3 pyramidal cell layer (arrowheads in H) compared with saline-injected mice (G). In contrast, neuronal loss was undetectable in PPT-A(−/−) mice treated with the same dose of kainate (I). (Scale bars: 125 μm, A–F; 80 μm, G and H.)
Table 2.
Relationship between PPT-A genotype and neuronal damage after kainate SE
| Damage scores | Hematoxylin & eosin
|
Cresyl violet
|
||
|---|---|---|---|---|
| PPT-A +/+ n = 10 | PPT-A −/−n = 6 | PPT-A +/+ n = 6 | PPT-A −/−n = 3 | |
| 0 | 2 (20%) | 4 (67%) | 2 (33%) | 2 (67%) |
| 1 | 1 (10%) | 1 (17%) | 0 (0%) | 0 (0%) |
| 2 | 0 (0%) | 1 (17%) | 0 (0%) | 1 (33%) |
| 3 | 3 (30%) | 0 (0%) | 1 (17%) | 0 (0%) |
| 4 | 4 (40%) | 0 (0%) | 3 (50%) | 0 (0%) |
Neuronal damage or loss were assessed 3 days (hematoxylin & eosin staining) and 7 days (cresyl violet staining) after kainate injection (35 mg/kg). Neuronal damage was scored (13) as follows: 0, no damage; 1, occasional injured neurons in CA1 or CA3; 2, small area (<10%) with neuronal damage or loss in CA1 or CA3; 3, greater area (10–50%) of neuronal damage or loss in CA1 or CA3; 4, extended (>50%) neuronal loss or damage in both CA1 and CA3.
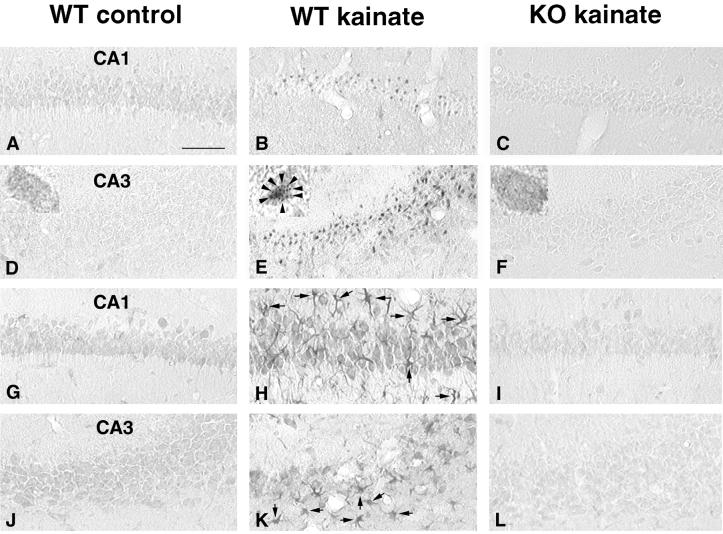
Seizures are reported to cause both necrotic and apoptotic neuronal death in the hippocampus (19–21). To investigate the mode of cell death induced by kainate SE in PPT-A(+/+) and PPT-A(−/−) mice, we screened injured neurons with the TUNEL method, which identifies fragmented double-stranded DNA, (13, 17, 24). Three days after injection of kainate (35 mg/kg) in PPT-A(+/+) mice (n = 3), we detected significant numbers of TUNEL-stained neurons in the CA1 subfield (Fig. 3B), a small number of labeled neurons in the CA3 region (Fig. 3E), and a few in the hilus. In contrast, we found no TUNEL-stained neurons in the hippocampus of three PPT-A(−/−) mice (Fig. 3 C and F), or in uninjected or saline-injected controls (Fig. 3 A and D). As the TUNEL method is not a specific indicator of apoptosis, we used EM to confirm the morphological appearance of TUNEL-positive cells. In PPT-A(+/+) mice 3 days after kainate injection, EM revealed that a few dying neurons (7 ± 2% of dying neurons in CA1 subfield) at the CA1-CA2 junction showed an apoptotic morphology as described by Portera-Cailliau et al. (16), i.e., compacting of chromatin into large round clumps with preservation of plasma membrane continuity, relative integrity of organelles, and cell body shrinkage (Fig. 3H). This feature could be distinguished from the signs of necrosis in the rest of CA1 and CA3, including overt swelling, cytolysis, and pyknotic nucleus with irregular contour of the chromatin (Fig. 3I). By contrast, the ultrastructural appearance of the cytoplasm and nucleus of CA1 and CA3 neurons in kainate-treated PPT-A(−/−) mice was normal (Fig. 3J) and not distinguishable from that observed in uninjected or saline-injected mice (Fig. 3G). Together, these results indicate that disruption of the PPT-A gene imparts resistance to kainate-induced necrosis and apoptosis.
Figure 3.
Comparison of kainate-induced apoptosis in the hippocampus in PPT-A(+/+) (WT) and PPT-A(−/−) (KO) mice. (A-F) TUNEL staining. None was apparent in saline-treated controls (A and D). Large numbers of TUNEL-positive neurons were dispersed throughout the CA1 layer (B), and significant numbers were scattered in the CA3 subfield (E) of PPT-A(+/+) mice 3 days after injection of kainate (35 mg/kg). No TUNEL-positive neurons were observed in PPT-A(−/−) mice treated with the same dose of kainate (C and F). (G–J) Electron micrographs of hippocampal neurons in PPT-A(+/+) and PPT-A(−/−) mice 3 days after injection of kainate (35 mg/kg). (G) A normal CA1 pyramidal cell of an untreated mouse. (H) In kainate-treated PPT-A(+/+) mice, some CA1 neurons (area adjacent to CA2) displayed apoptotic-like features such as chromatin condensation into a few round clumps (arrows in H) and condensation of a relatively intact cytoplasm with preservation of plasma membrane. (I) Necrotic features, i.e., numerous small vacuoles throughout the cytoplasm as well as disruption of plasma membrane and a pyknotic nucleus with irregular contour of the chromatin clumps (arrowheads in I) were seen in the rest of CA1 (and in CA3, not shown) of PPT-A(+/+) mice 3 days after injection of kainate. (J) A CA1 neuron from a PPT-A(−/−) mouse 3 days after injection of kainate exhibiting normal ultrastructural features. (Scale bars: 125 μm, A–F; 10 μm, G–J.)
In PPT-A(−/−) Mice, Kainate Does Not Induce Expression of Cell Death Genes.
One possible explanation for the differential kainate susceptibility of the two groups of mice is that the PPT-A gene is an important intracellular mediator of kainate excitotoxicity. To test this hypothesis, we examined the effect of kainate SE on expression of the bax gene. In untreated mice there were a few, faintly bax-immunoreactive (bax-ir) cells in CA3, CA1, and the dentate hilus (Fig. 4 A and D). In these cells, bax immunoreactivity was distributed throughout the cytosol (Fig. 4D, Inset). By 3 days after the injection of kainate (35 mg/kg), the number of bax-ir pyramidal neurons in PPT-A(+/+) mice (n = 6) increased by 37 ± 7% in CA1 and 69 ± 8% in CA3 (Fig. 4 B and E) compared with corresponding areas of untreated wild-type mice (Fig. 4 A and D). The strong bax immunoreactivity in these cells had a punctate distribution (Fig. 4E, Inset), suggesting that bax was redistributed, possibly to mitochondria, and activated, as has been reported during induction of cell death (25–27). In contrast, after kainate treatment in the PPT-A(−/−) mice (n = 3) there was no significant increase either in the number of bax-ir cells or in the density and distribution of bax-ir staining (Fig. 4 C and F). Because activation of bax regulates caspase 3 expression (26, 27) we also tested the effect of kainate on caspase 3-ir. Consistent with studies in the rat, in untreated wild-type mice, we found faint caspase 3-ir in the pyramidal cell layers or hilus (Fig. 4 G and J) (28). Three days after injection of kainate (n = 6), we observed a pronounced increase in caspase 3-ir in pyramidal neurons in both CA3 (79 ± 11%) and CA1 (83 ± 9%) (Fig. 4 H and K). Interestingly, large numbers of glial cells (as judged by an astrocyte-like morphology) were stained with caspase 3 throughout the hippocampus (Fig, 4 H and K). These results are consistent with the activation by kainate of a cell death program, including expression of caspase 3 in neurons and astrocytes (29), in spite of the necrotic EM appearance of many cells. In contrast, only a trace amount of caspase 3-ir, not significantly different from that in uninjected or saline-injected mice (Fig. 4 G and J), was found in CA3b and CA1 (Fig. 4 I and L) in kainate-treated PPT-A(−/−) mice (n = 3). These data demonstrate that disruption of the PPT-A gene suppressed kainate-induced activation of two intracellular regulators of cell death (30, 31) in the hippocampus and that mice carrying a disruption of the gene that encodes SP/NKA have significantly reduced behavioral seizures and neuronal death after systemic injection of kainate.
Figure 4.
Kainate-induced expression of bax and caspase 3 in PPT-A(+/+) and PPT-A(−/−) mice. (A-F) Immunocytochemical analysis of bax. In saline-treated controls (A and D), there were few bax-ir neurons, and bax-ir was distributed throughout the cytosol (Inset in D, ×100). Expression of bax was markedly increased in the CA1 (B) and CA3 (E) regions in kainate-treated PPT-A(+/+) mice. Bax-ir was redistributed in punctate fashion (arrowhead in Inset in E, ×100) in PPT-A(+/+) mice 3 days after injection of kainate (35 mg/kg). In contrast, no increase in bax-ir was found in hippocampi (C and F), and bax-ir was not redistributed (Inset in F, ×100) in PPT-A(−/−) mice treated with the same dose of kainate. (G–L) Immunocytochemical analysis of caspase 3-ir. In the saline-injected mice, a small amount of caspase 3-ir was detected in hippocampus (G and J). Three days after injection of kainate (35 mg/kg), there was a dramatic increase in caspase 3-ir in neurons through the entire pyramidal layer of PPT-A(+/+) mice (H and K); there was also a dramatic increase in caspase 3-ir in glial cells over the entire hippocampus (arrows in H and K). By contrast, there was only minimal induction of caspase 3-ir in CA1 (I) and CA3 (L) in PPT-A(−/−) mice. (Scale bar: 125 μm).
Discussion
In the present study, we have used mice deficient in the PPT-A gene encoding SP/NKA to examine the effect of SP/NKA loss on chemical-induced limbic seizures and neuronal injury. Our results demonstrate, in these mice, a significant reduction of both kainate-induced and PTZ-induced behavioral seizures and a decrease in kainate-induced hippocampal neuronal death.
Linking epileptic seizures to an identified gene adds to current insights into the genetic basis for SE. Our studies support a proconvulsant role for the PPT-A gene, because PPT-A deficiency significantly reduced not only PTZ-induced, but also kainate-induced seizures. Although NKA is colocalized with SP in most areas, it is present in comparatively minute quantities, with little or no NK2 receptor expression in brain (32), whereas NK1 receptors are abundant, making it likely that these effects are mediated by SP. These results are consistent with previous observations that intrahippocampal injection of SP triggered SE whereas injection of SP antagonists prevented or stopped it (9). Taken together, the present results indicated that SP is an important effector in the development of SE.
Various mechanisms have been suggested for the excitatory effects of SP. SP has been shown to excite neurons by suppressing an inwardly rectifying potassium current (33) and by suppressing the M- and N-type calcium currents (34, 35), which can lead to a reduction of Ca2+-activated potassium currents and the resulting after-hyperpolarization (36). Because seizures often result from excess release of the excitatory amino acid glutamate from presynaptic terminals (2–4), the contribution of SP in the present study may involve interaction in the processing of excitatory information. This hypothesis is supported by previous evidence that SP enhances glutamate currents mediated by NMDA receptors (5, 6, 37). Most importantly, SP enhances release of glutamate in the hippocampus (9). Thus, the reduction of chemically induced seizures in PPT-A(−/−) mice might be caused by the extinction of SP-mediated enhancement of glutamate release and glutamate responses.
Although it is unclear whether the reduction of hippocampal neuronal injury is caused by a neuroprotective effect of SP or a lessening of seizure activity, evidence obtained from the present study suggests that lack of the PPT-A gene almost eliminated kainate-induced hippocampal neuronal death. PPT-A deficiency also led to decrease in expression of bax and caspase 3. These findings indicate that SP could be involved in a seizure-induced cell death pathway involving bax and caspases. SP, while increasing intracellular Ca2+ (6) and enhancing glutamate responses (7, 8) or glutamate release (9, 38), may induce expression and activation of bax (39) and caspase 3 (31). Activation of bax may trigger the release of cytochrome c and other proteins from the mitochondria and induce caspase activation in cytosol (26, 27). The induction of caspase 3 may activate DNA and nuclear fragmentation, promoting cell death (40).
It is interesting that in kainate-treated wild-type mice, many of the features classically attributed to apoptosis were seen in neurons, which were clearly necrotic by EM criteria. These CA3 and CA1 neurons showed not only increased expression but also redistribution of bax, they displayed double-stranded DNA breaks (41) and expressed the key apoptotic enzyme, caspase 3 (28, 31), in large amounts. Several recent reports (42, 43) have noted the expression of genes involved in the apoptotic cascade in cells that appear necrotic morphologically, suggesting either that both processes can be induced simultaneously in the same cell or that the induction of cell suicide programs eventually can result in a necrotic morphology.
Our results indicate an important role for a SP/NKA-mediated signaling pathway in the pathogenesis of kainate SE and excitotoxicity. They also suggest that tachykinin antagonists may represent a novel category of drugs in treating diseases involving neuronal degeneration and seizures.
Acknowledgments
We thank R. Baldwin and D. Shin for technical assistance. This work was supported by Research Grant NS 13515 from the National Institute of Neurological Disorders and Stroke (C.G.W.), by Grant NS14627 from the National Institute of Neurological Disorders and Stroke (A.I.B.), and by the Clinical Investigator Development Award NS01792 from the National Institutes of Health (R.S.), and by the Research Service of the Veterans Administration (C.G.W.).
Abbreviations
- bax-ir
bax immunoreactive
- EM
electron microscopy
- NK
neurokinin
- PPT-A
preprotachykinin A
- PTZ
pentylenetetrazole
- SE
status epilepticus
- SP
substance P
- TUNEL
terminal transferase-mediated biotinylated-UTP nick end-labeling
References
- 1.De Lorenzo R J. In: Seizures and Epilepsy in the Elderly. Rowan A J, Ramsey R E, editors. Boston: Butterworth–Heinemann; 1997. pp. 191–205. [Google Scholar]
- 2.Olney J W, Collins R C, Sloviter R S. Adv Neurol. 1986;44:857–877. [PubMed] [Google Scholar]
- 3.Meldrum B S, Garthwaite J. Trends Pharmacol Sci. 1990;11:379–387. doi: 10.1016/0165-6147(90)90184-a. [DOI] [PubMed] [Google Scholar]
- 4.Coyle J T, Puttfarcken P. Science. 1993;262:689–695. doi: 10.1126/science.7901908. [DOI] [PubMed] [Google Scholar]
- 5.Randic M, Hecimovic H, Ryu P D. Neurosci Lett. 1990;117:74–80. doi: 10.1016/0304-3940(90)90122-p. [DOI] [PubMed] [Google Scholar]
- 6.Rusin K I, Bleakman D, Chard P S, Randic M, Miller R J. J Neurochem. 1993;60:952–960. doi: 10.1111/j.1471-4159.1993.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 7.Urban L, Thompson S W, Dray A. Trends Neurosci. 1994;17:432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 8.Cumberbatch M J, Chizh B A, Headley P M. Br J Pharmacol. 1995;115:1005–1012. doi: 10.1111/j.1476-5381.1995.tb15911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Mazarati A M, Katsumori H, Sankar R, Wasterlain C G. Proc Natl Acad Sci USA. 1999;96:5286–5291. doi: 10.1073/pnas.96.9.5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zachrisson O, Lindefors N, Brene S A. Mol Brain Res. 1998;60:291–295. doi: 10.1016/s0169-328x(98)00191-0. [DOI] [PubMed] [Google Scholar]
- 11.Yu Z F, Cheng G J, Huang X F, Li K Y, Cao X D. NeuroReport. 1997;8:2117–2119. doi: 10.1097/00001756-199707070-00006. [DOI] [PubMed] [Google Scholar]
- 12.Cao Y Q, Mantyh P W, Carlson E J, Gillespie A M, Epstein C J, Basbaum A I. Nature (London) 1998;392:390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 13.Morrison R S, Wenzel H J, Kinoshita Y, Robbins C A, Donehower L A, Schwartzkroin P A. J Neurosci. 1996;16:1337–1345. doi: 10.1523/JNEUROSCI.16-04-01337.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.West M J. Neurobiol Aging. 1993;14:275–285. doi: 10.1016/0197-4580(93)90112-o. [DOI] [PubMed] [Google Scholar]
- 15.Liu H, Mantyh P W, Basbaum A I. Nature (London) 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- 16.Portera-Cailliau C, Price D L, Martin L J. J Comp Neurol. 1997;378:70–87. [PubMed] [Google Scholar]
- 17.Wood K A, Dipasquale B, Youle R J. Neuron. 1993;11:621–632. doi: 10.1016/0896-6273(93)90074-2. [DOI] [PubMed] [Google Scholar]
- 18.Ferkany J W, Zaczek R, Coyle J T. Nature (London) 1984;308:561–562. doi: 10.1038/308561a0. [DOI] [PubMed] [Google Scholar]
- 19.Ben-Ari Y, Repressa A, Tremblay E, Nitecka L. Adv Exp Med Biol. 1986;203:647–657. doi: 10.1007/978-1-4684-7971-3_49. [DOI] [PubMed] [Google Scholar]
- 20.Sloviter R S, Dean E, Sollas A L, Goodman J H. J Comp Neurol. 1996;366:516–533. doi: 10.1002/(SICI)1096-9861(19960311)366:3<516::AID-CNE10>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 21.Sankar R, Shin D, Liu H, Mazarati A, de Vasconcelos A P, Wasterlain C G. J Neurosci. 1998;18:8382–8393. doi: 10.1523/JNEUROSCI.18-20-08382.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang D D, Kuan C Y, Whitmarsh A J, Rincon M, Zheng T S, Davis R J, Rakic P, Flavell R A. Nature (London) 1997;389:865–870. doi: 10.1038/39899. [DOI] [PubMed] [Google Scholar]
- 23.Choi D W. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Symonds H, Krall L, Remington L, Saenz-Robles M, Lowe S, Jaxks T. Cell. 1994;78:703–711. doi: 10.1016/0092-8674(94)90534-7. [DOI] [PubMed] [Google Scholar]
- 25.Wolter K G, Hsu Y T, Smith C L, Nechushtan A, Xi X G, Youle R J. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosse T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B, Borner C. Nature (London) 1998;391:496–499. doi: 10.1038/35160. [DOI] [PubMed] [Google Scholar]
- 27.Marzo I, Brenner C, Zamzami N, Jurgensmeier J M, Susin S A, Vieira S L A, Prevost M-C, Xie Z, Matsuyama S, Reed J C, Kroemer G. Science. 1998;281:2027–2031. doi: 10.1126/science.281.5385.2027. [DOI] [PubMed] [Google Scholar]
- 28.Chen J, Nagayama T, Jin K, Steler R A, Zhu R L, Graham S H, Smon R P. J Neurosci. 1998;18:4914–4928. doi: 10.1523/JNEUROSCI.18-13-04914.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keane R W. J Neurosci Res. 1997;48:168–180. doi: 10.1002/(sici)1097-4547(19970415)48:2<168::aid-jnr9>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Deckwerth T L, Elliott J L. Neuron. 1996;17:401–411. doi: 10.1016/s0896-6273(00)80173-7. [DOI] [PubMed] [Google Scholar]
- 31.Gillardon F, Bottiger B, Schmitz B, Zimmermann M, Hossmann K A. Mol Brain Res. 1997;50:16–22. doi: 10.1016/s0169-328x(97)00162-9. [DOI] [PubMed] [Google Scholar]
- 32.Tsuchida K, Shigemoto R, Yokoto Y, Nakanishi S. Eur J Pharmacol. 1990;193:751–757. doi: 10.1111/j.1432-1033.1990.tb19396.x. [DOI] [PubMed] [Google Scholar]
- 33.Stanfield P R, Nakajima Y, Yamaguchi K. Nature (London) 1985;315:498–510. doi: 10.1038/315498a0. [DOI] [PubMed] [Google Scholar]
- 34.Bley K R, Tsien R W. Neuron. 1990;4:379–391. doi: 10.1016/0896-6273(90)90050-p. [DOI] [PubMed] [Google Scholar]
- 35.Shapiro M S, Hille B. Neuron. 1993;10:11–20. doi: 10.1016/0896-6273(93)90237-l. [DOI] [PubMed] [Google Scholar]
- 36.Adams P R, Jones S W, Pennefather P, Brown D A, Koch C, Lancaster B. J Exp Biol. 1986;124:259–285. doi: 10.1242/jeb.124.1.259. [DOI] [PubMed] [Google Scholar]
- 37.Lieberman D N, Mody I. J Neurophysiol. 1998;80:113–119. doi: 10.1152/jn.1998.80.1.113. [DOI] [PubMed] [Google Scholar]
- 38.Heath M J, Womack M D, MacDermott A B. J Neurophysiol. 1994;72:1192–1198. doi: 10.1152/jn.1994.72.3.1192. [DOI] [PubMed] [Google Scholar]
- 39.Gillardon F, Wickert H, Zimmermann M. Neurosci Lett. 1995;192:85–88. doi: 10.1016/0304-3940(95)11619-8. [DOI] [PubMed] [Google Scholar]
- 40.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. Nature (London) 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 41.Pastorino J G, Chen S-T, Tafani M, Snyder J W, Farber J L. J Biol Chem. 1998;273:7770–7775. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 42.Fujikawa D G, Shinmei S S, Cai B. Eur J Neurosci. 1999;11:1605–1614. doi: 10.1046/j.1460-9568.1999.00573.x. [DOI] [PubMed] [Google Scholar]
- 43.van Lookeren Campagne M, Lucassen P J, Vermeulen J P, Balazs R. Eur J Neurosci. 1995;7:1627–1640. doi: 10.1111/j.1460-9568.1995.tb01158.x. [DOI] [PubMed] [Google Scholar]