Abstract
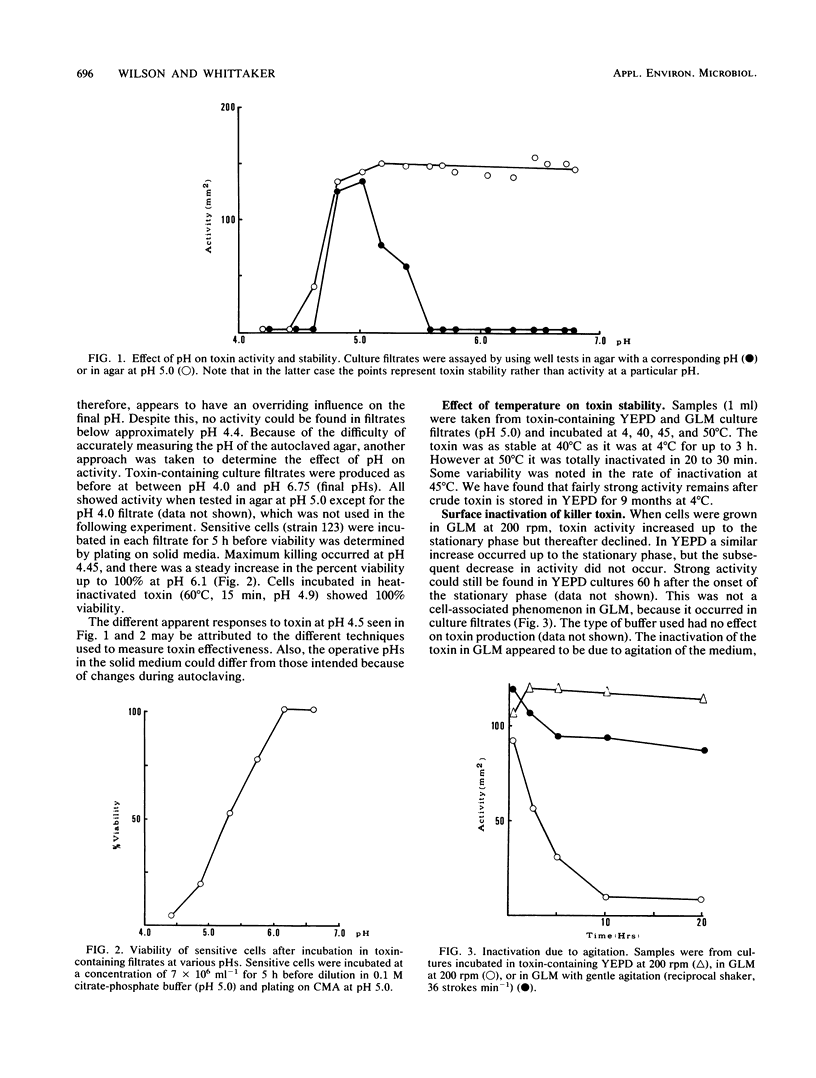
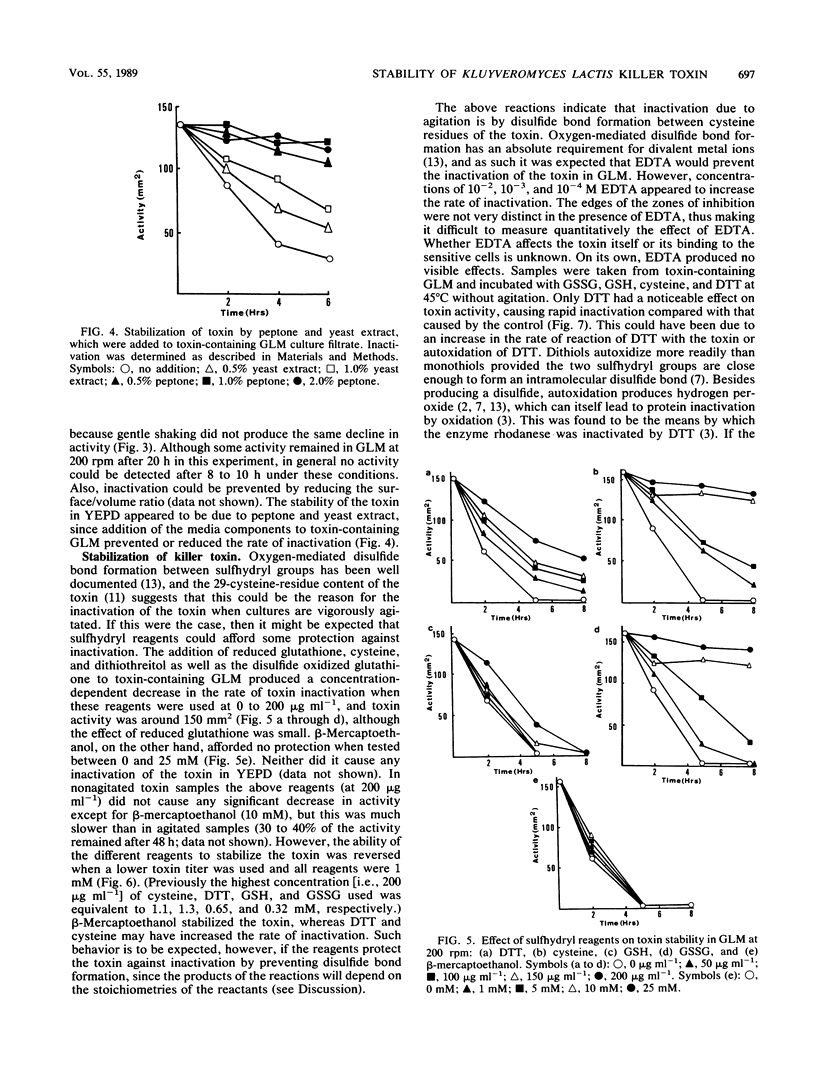
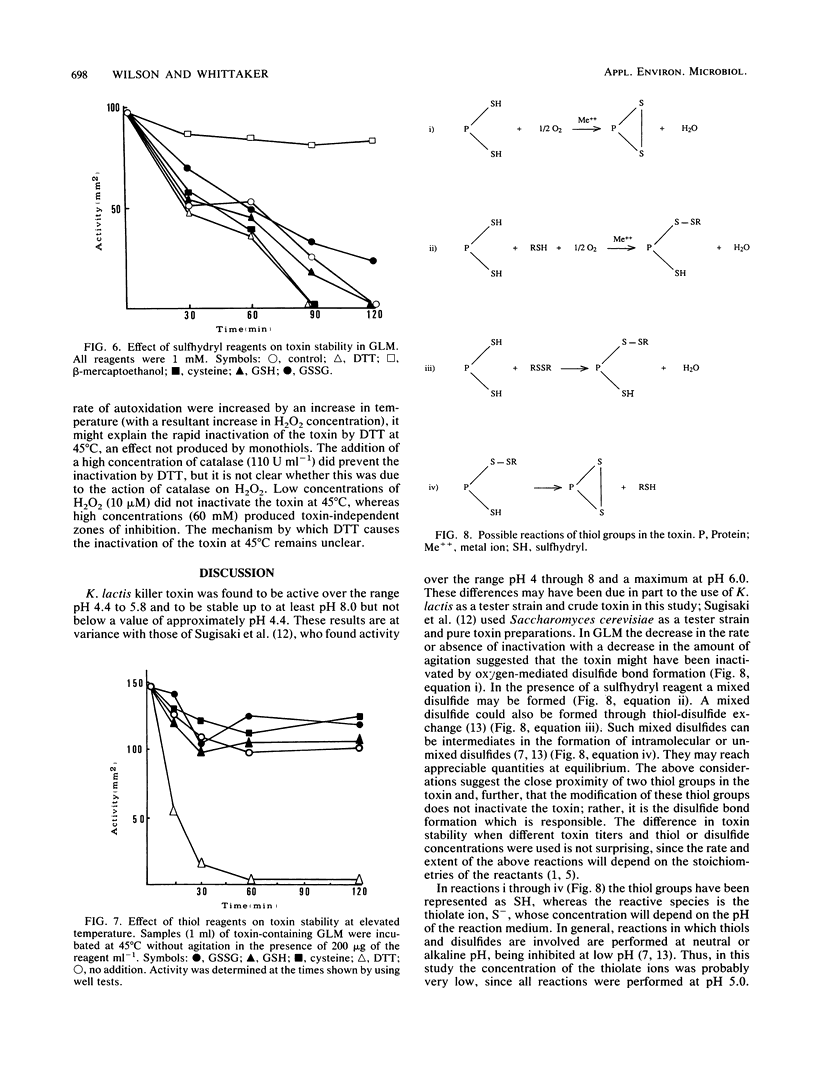
A number of physical parameters determining the activity and stability of the killer toxin produced by the yeast Kluyveromyces lactis have been investigated. The toxin was active over a relatively narrow pH range of 4.4 to 5.8, with a maximum at the lower end of the range. However, it was stable up to at least pH 8.0 but appeared to be irreversibly inactivated below pH 4.4. The toxin was stable at 40°C but rapidly inactivated at 50°C. Strong agitation caused the inactivation of the toxin in one medium but not another; this seemed to be due to oxygen-mediated disulfide bond formation, which could be prevented by sulfhydryl protecting agents.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradshaw R. A., Kanarek L., Hill R. L. The preparation, properties, and reactivation of the mixed disulfide derivative of egg white lysozyme and L-cystine. J Biol Chem. 1967 Sep 10;242(17):3789–3798. [PubMed] [Google Scholar]
- Cavallini D., De Marco C., Dupré S. Luminol chemiluminescence studies of the oxidation of cysteine and other thiols to disulfides. Arch Biochem Biophys. 1968 Mar 20;124(1):18–26. doi: 10.1016/0003-9861(68)90299-3. [DOI] [PubMed] [Google Scholar]
- Costa M., Pecci L., Pensa B., Cannella C. Hydrogen peroxide involvement in the rhodanese inactivation by dithiothreitol. Biochem Biophys Res Commun. 1977 Sep 23;78(2):596–603. doi: 10.1016/0006-291x(77)90221-2. [DOI] [PubMed] [Google Scholar]
- Drenth J., Low B. W., Richardson J. S., Wright C. S. The toxin-agglutinin fold. A new group of small protein structures organized around a four-disulfide core. J Biol Chem. 1980 Apr 10;255(7):2652–2655. [PubMed] [Google Scholar]
- Gunge N., Tamaru A., Ozawa F., Sakaguchi K. Isolation and characterization of linear deoxyribonucleic acid plasmids from Kluyveromyces lactis and the plasmid-associated killer character. J Bacteriol. 1981 Jan;145(1):382–390. doi: 10.1128/jb.145.1.382-390.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa O., Sakaguchi K., Gunge N. Curing of the killer deoxyribonucleic acid plasmids of Kluyveromyces lactis. J Bacteriol. 1981 Dec;148(3):988–990. doi: 10.1128/jb.148.3.988-990.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Boyd A. The killer toxin of Kluyveromyces lactis: characterization of the toxin subunits and identification of the genes which encode them. EMBO J. 1986 Aug;5(8):1995–2002. doi: 10.1002/j.1460-2075.1986.tb04455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark M. J., Mileham A. J., Romanos M. A., Boyd A. Nucleotide sequence and transcription analysis of a linear DNA plasmid associated with the killer character of the yeast Kluyveromyces lactis. Nucleic Acids Res. 1984 Aug 10;12(15):6011–6030. doi: 10.1093/nar/12.15.6011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugisaki Y., Gunge N., Sakaguchi K., Yamasaki M., Tamura G. Characterization of a novel killer toxin encoded by a double-stranded linear DNA plasmid of Kluyveromyces lactis. Eur J Biochem. 1984 Jun 1;141(2):241–245. doi: 10.1111/j.1432-1033.1984.tb08183.x. [DOI] [PubMed] [Google Scholar]


