Abstract
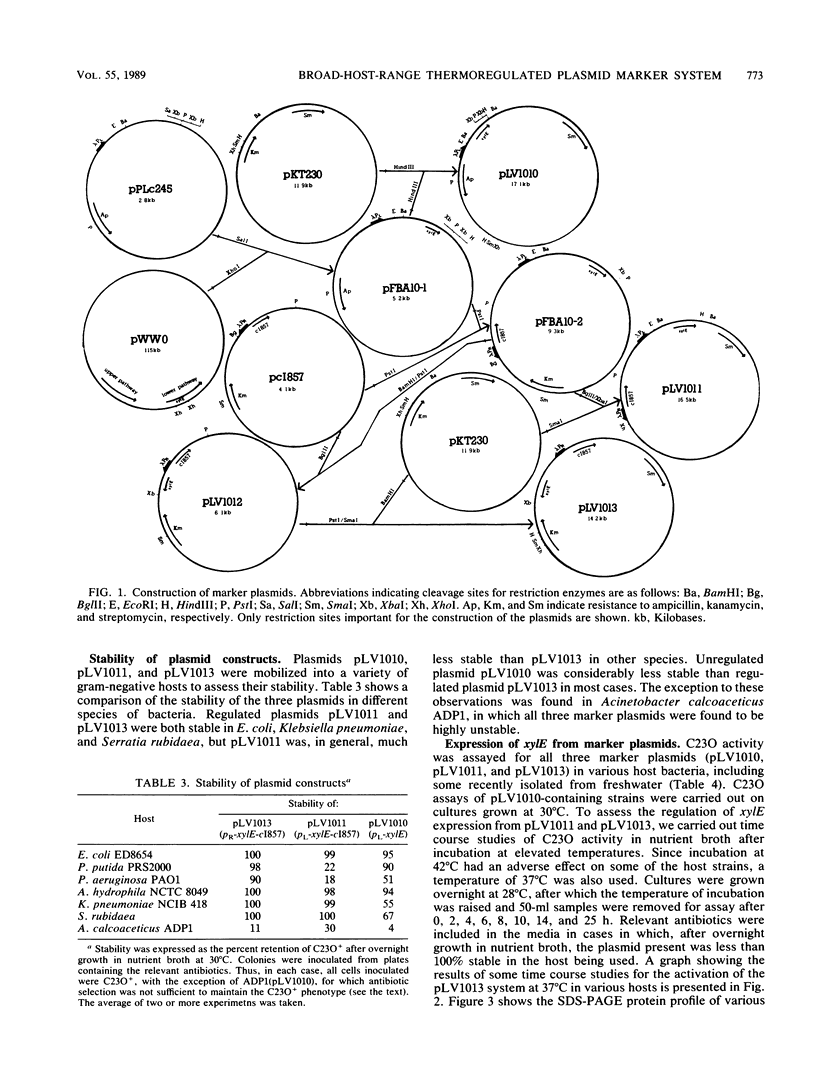
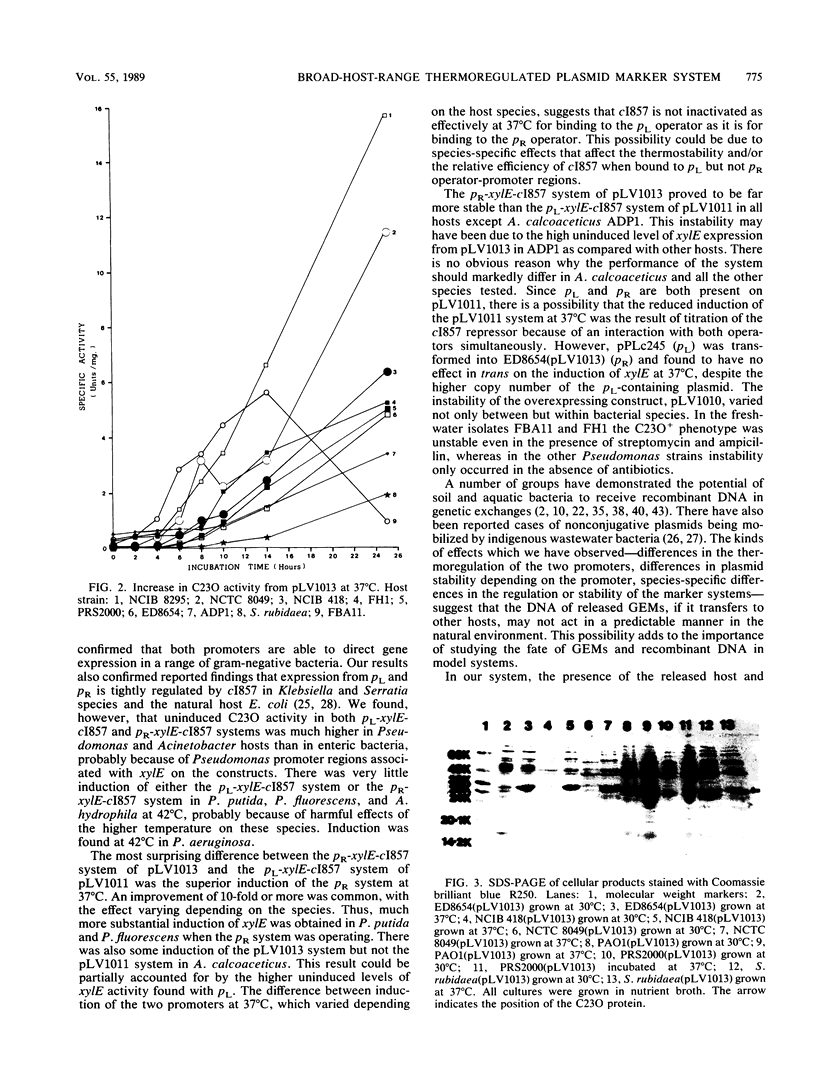
Plasmid systems with unique markers were constructed to assess the fate of recombinant DNA and genetically manipulated bacteria in soil and freshwater model environments. On such constructs the marker gene, xylE (for catechol 2,3-dioxygenase), is expressed from the lambda promoter pL or pR, each of which is controlled by the temperature-sensitive lambda repressor c1857. Combinations of these elements were cloned into the broad-host-range plasmid pKT230 to form pLV1010 (pL-xylE), pLV1011 (pL-xylE-c1857), and pLV1013 (pR-xylE-c1857). The recombinant plasmids were introduced into different gram-negative bacteria. The thermoregulated system of pLV1013 functioned well in a range of species, with xylE induction being readily achieved by elevation of the temperature from 28 to 37 degrees C. There was a difference in the induction of catechol 2,3-dioxygenase activity, depending on whether xylE was expressed from pL (pLV1011) or pR (pLV1013). Our observations on testing the different systems in a number of hosts suggest that genes carried by the DNA of genetically engineered microorganisms may not be expressed in a predictable manner following transfer from the release host to other species.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagdasarian M., Lurz R., Rückert B., Franklin F. C., Bagdasarian M. M., Frey J., Timmis K. N. Specific-purpose plasmid cloning vectors. II. Broad host range, high copy number, RSF1010-derived vectors, and a host-vector system for gene cloning in Pseudomonas. Gene. 1981 Dec;16(1-3):237–247. doi: 10.1016/0378-1119(81)90080-9. [DOI] [PubMed] [Google Scholar]
- Bale M. J., Fry J. C., Day M. J. Plasmid transfer between strains of Pseudomonas aeruginosa on membrane filters attached to river stones. J Gen Microbiol. 1987 Nov;133(11):3099–3107. doi: 10.1099/00221287-133-11-3099. [DOI] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Stewart D. J., McKern N. M., Peterson J. E. Expression of pili from Bacteroides nodosus in Pseudomonas aeruginosa. J Bacteriol. 1986 Nov;168(2):574–580. doi: 10.1128/jb.168.2.574-580.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman G. G. The fermentation of sucrose by Aerobacter aerogenes. Biochem J. 1947;41(3):389–398. doi: 10.1042/bj0410389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genthner F. J., Chatterjee P., Barkay T., Bourquin A. W. Capacity of aquatic bacteria to act as recipients of plasmid DNA. Appl Environ Microbiol. 1988 Jan;54(1):115–117. doi: 10.1128/aem.54.1.115-117.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godwin D., Slater J. H. The influence of the growth environment on the stability of a drug resistance plasmid in Escherichia coli K12. J Gen Microbiol. 1979 Mar;111(1):201–210. doi: 10.1099/00221287-111-1-201. [DOI] [PubMed] [Google Scholar]
- Grinter N. J. A broad-host-range cloning vector transposable to various replicons. Gene. 1983 Jan-Feb;21(1-2):133–143. doi: 10.1016/0378-1119(83)90155-5. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas D., Holloway B. W. R factor variants with enhanced sex factor activity in Pseudomonas aeruginosa. Mol Gen Genet. 1976 Mar 30;144(3):243–251. doi: 10.1007/BF00341722. [DOI] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway B. W. Genetics of Pseudomonas. Bacteriol Rev. 1969 Sep;33(3):419–443. doi: 10.1128/br.33.3.419-443.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hughes V. M., Datta N. Conjugative plasmids in bacteria of the 'pre-antibiotic' era. Nature. 1983 Apr 21;302(5910):725–726. doi: 10.1038/302725a0. [DOI] [PubMed] [Google Scholar]
- Jones J. G., Gardener S., Simon B. M., Pickup R. W. Antibiotic resistant bacteria in Windermere and two remote upland tarns in the English Lake District. J Appl Bacteriol. 1986 May;60(5):443–453. doi: 10.1111/j.1365-2672.1986.tb05090.x. [DOI] [PubMed] [Google Scholar]
- Jones J. G., Gardener S., Simon B. M., Pickup R. W. Factors affecting the measurement of antibiotic resistance in bacteria isolated from lake water. J Appl Bacteriol. 1986 May;60(5):455–462. doi: 10.1111/j.1365-2672.1986.tb05091.x. [DOI] [PubMed] [Google Scholar]
- Juni E. Interspecies transformation of Acinetobacter: genetic evidence for a ubiquitous genus. J Bacteriol. 1972 Nov;112(2):917–931. doi: 10.1128/jb.112.2.917-931.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen J. E., Gerdes K., Light J., Molin S. Low-copy-number plasmid-cloning vectors amplifiable by derepression of an inserted foreign promoter. Gene. 1984 Apr;28(1):45–54. doi: 10.1016/0378-1119(84)90086-6. [DOI] [PubMed] [Google Scholar]
- Leemans R., Remaut E., Fiers W. A broad-host-range expression vector based on the pL promoter of coliphage lambda: regulated synthesis of human interleukin 2 in Erwinia and Serratia species. J Bacteriol. 1987 May;169(5):1899–1904. doi: 10.1128/jb.169.5.1899-1904.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini P., Fertels S., Nave D., Gealt M. A. Mobilization of plasmid pHSV106 from Escherichia coli HB101 in a laboratory-scale waste treatment facility. Appl Environ Microbiol. 1987 Apr;53(4):665–671. doi: 10.1128/aem.53.4.665-671.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson P., Gealt M. A. Isolation of indigenous wastewater bacterial strains capable of mobilizing plasmid pBR325. Appl Environ Microbiol. 1986 May;51(5):904–909. doi: 10.1128/aem.51.5.904-909.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muyzer G., de Bruyn A. C., Schmedding D. J., Bos P., Westbroek P., Kuenen G. J. A Combined Immunofluorescence-DNA-Fluorescence Staining Technique for Enumeration of Thiobacillus ferrooxidans in a Population of Acidophilic Bacteria. Appl Environ Microbiol. 1987 Apr;53(4):660–664. doi: 10.1128/aem.53.4.660-664.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai C., Kagamiyama H., Nozaki M., Nakazawa T., Inouye S., Ebina Y., Nakazawa A. Complete nucleotide sequence of the metapyrocatechase gene on the TOI plasmid of Pseudomonas putida mt-2. J Biol Chem. 1983 Mar 10;258(5):2923–2928. [PubMed] [Google Scholar]
- O'Farrell P. H., Kutter E., Nakanishi M. A restriction map of the bacteriophage T4 genome. Mol Gen Genet. 1980;179(2):421–435. doi: 10.1007/BF00425473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Stanssens P., Fiers W. Inducible high level synthesis of mature human fibroblast interferon in Escherichia coli. Nucleic Acids Res. 1983 Jul 25;11(14):4677–4688. doi: 10.1093/nar/11.14.4677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remaut E., Tsao H., Fiers W. Improved plasmid vectors with a thermoinducible expression and temperature-regulated runaway replication. Gene. 1983 Apr;22(1):103–113. doi: 10.1016/0378-1119(83)90069-0. [DOI] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Evans W. C. The meta cleavage of catechol by Azotobacter species. 4-Oxalocrotonate pathway. Eur J Biochem. 1971 Jun 11;20(3):400–413. doi: 10.1111/j.1432-1033.1971.tb01406.x. [DOI] [PubMed] [Google Scholar]
- Saye D. J., Ogunseitan O., Sayler G. S., Miller R. V. Potential for transduction of plasmids in a natural freshwater environment: effect of plasmid donor concentration and a natural microbial community on transduction in Pseudomonas aeruginosa. Appl Environ Microbiol. 1987 May;53(5):987–995. doi: 10.1128/aem.53.5.987-995.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stotzky G., Babich H. Survival of, and genetic transfer by, genetically engineered bacteria in natural environments. Adv Appl Microbiol. 1986;31:93–138. doi: 10.1016/s0065-2164(08)70440-4. [DOI] [PubMed] [Google Scholar]
- TOTTER J. R., MOSELEY F. T. Influence of the concentration of iron on the production of fluorescin by Pseudomonas aeruginosa. J Bacteriol. 1953 Jan;65(1):45–47. doi: 10.1128/jb.65.1.45-47.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatcroft R., Williams P. A. Rapid methods for the study of both stable and unstable plasmids in Pseudomonas. J Gen Microbiol. 1981 Jun;124(2):433–437. doi: 10.1099/00221287-124-2-433. [DOI] [PubMed] [Google Scholar]
- Williams P. A., Murray K. Metabolism of benzoate and the methylbenzoates by Pseudomonas putida (arvilla) mt-2: evidence for the existence of a TOL plasmid. J Bacteriol. 1974 Oct;120(1):416–423. doi: 10.1128/jb.120.1.416-423.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeph L. R., Onaga M. A., Stotzky G. Transduction of Escherichia coli by bacteriophage P1 in soil. Appl Environ Microbiol. 1988 Jul;54(7):1731–1737. doi: 10.1128/aem.54.7.1731-1737.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]




