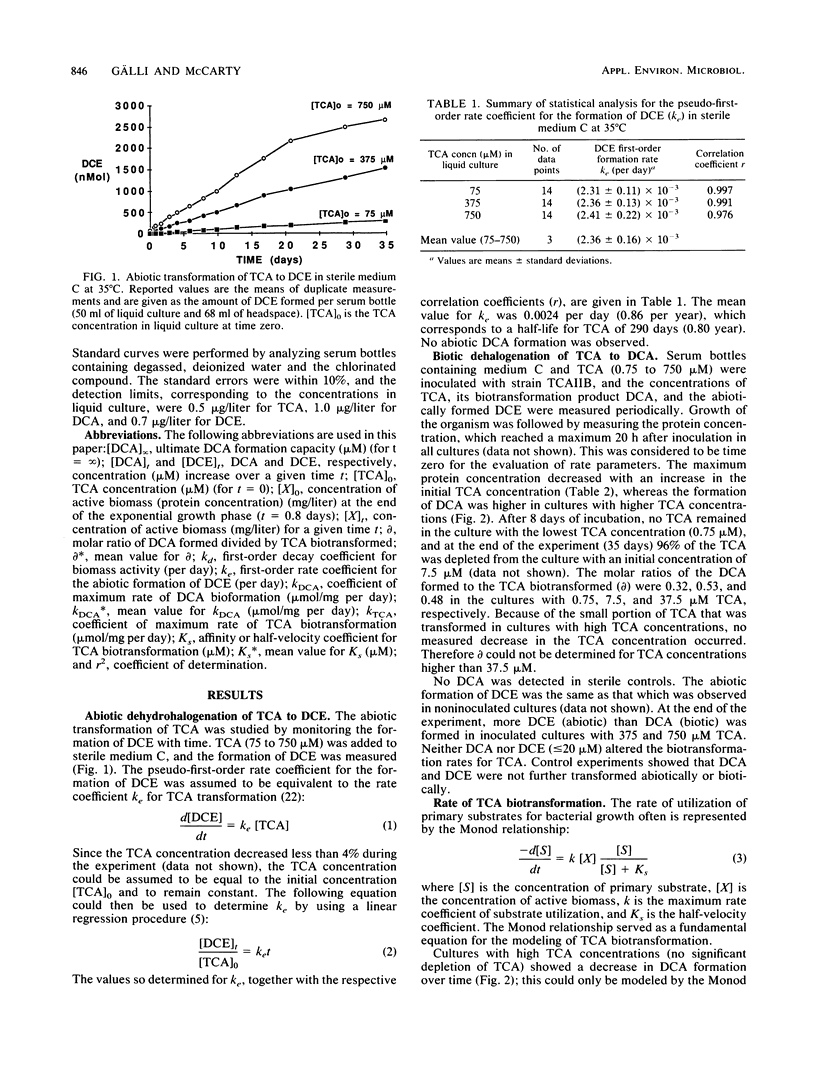
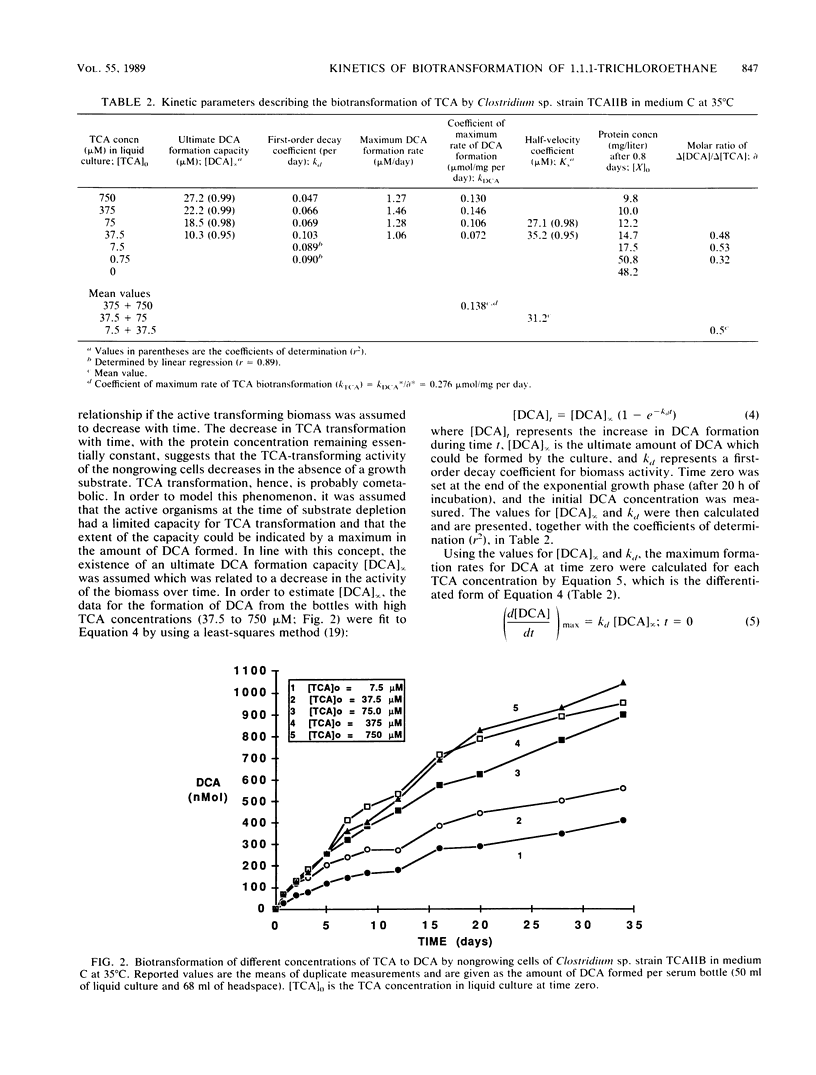
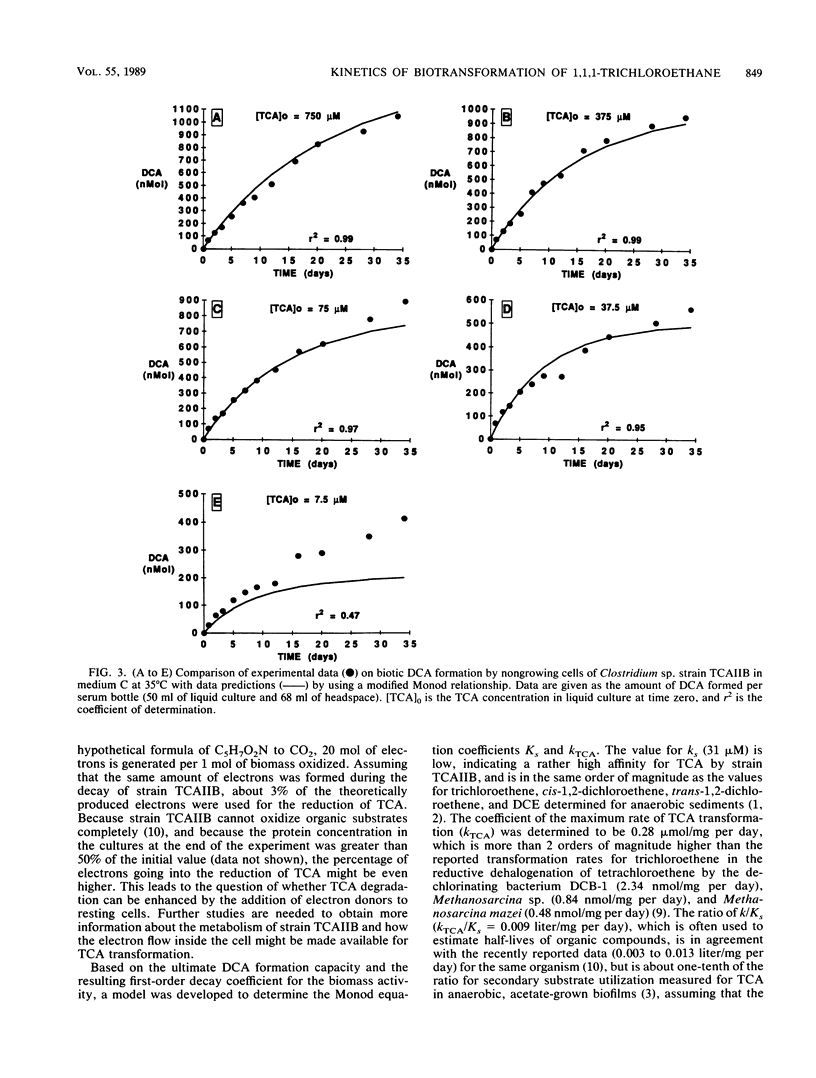
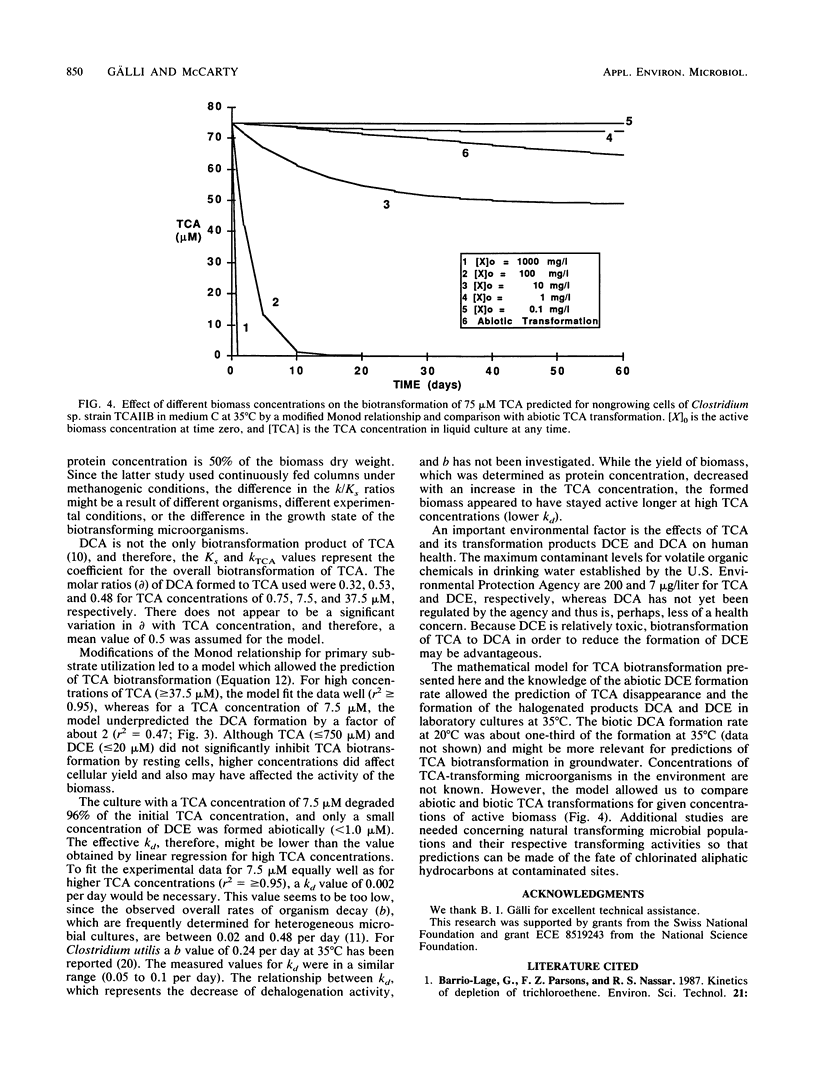
Abstract
Batch experiments were conducted to examine the effects of high concentrations of 1,1,1-trichloroethane (TCA) on the biotransformation of TCA by Clostridium sp. strain TCAIIB. The biotic dehalogenation of TCA to 1,1-dichloroethane by nongrowing cells was measured at 35 degrees C, and the data were used to obtain the kinetic parameters of the Monod relationship half-velocity coefficient Ks (31 microM) and the coefficient of maximum rate of TCA biotransformation (kTCA; 0.28 mumol per mg per day). The yield of biomass decreased with an increase in the TCA concentration, although TCA concentrations up to 750 microM did not completely inhibit bacterial growth. Also, kTCA was higher in the presence of high concentrations of TCA. A mathematical model based on a modified Monod equation was used to describe the biotransformation of TCA. The abiotic transformation of TCA to 1,1-dichloroethene was measured at 35 degrees C, and the first-order formation rate coefficient for 1,1-dichloroethene (ke) was determined to be 0.86 per year.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Fathepure B. Z., Nengu J. P., Boyd S. A. Anaerobic bacteria that dechlorinate perchloroethene. Appl Environ Microbiol. 1987 Nov;53(11):2671–2674. doi: 10.1128/aem.53.11.2671-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gälli R., McCarty P. L. Biotransformation of 1,1,1-trichloroethane, trichloromethane, and tetrachloromethane by a Clostridium sp. Appl Environ Microbiol. 1989 Apr;55(4):837–844. doi: 10.1128/aem.55.4.837-844.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson C. R., McConnell G. Chlorinated C1 and C2 hydrocarbons in the marine environment. Proc R Soc Lond B Biol Sci. 1975 May 20;189(1096):305–332. doi: 10.1098/rspb.1975.0059. [DOI] [PubMed] [Google Scholar]


