Abstract
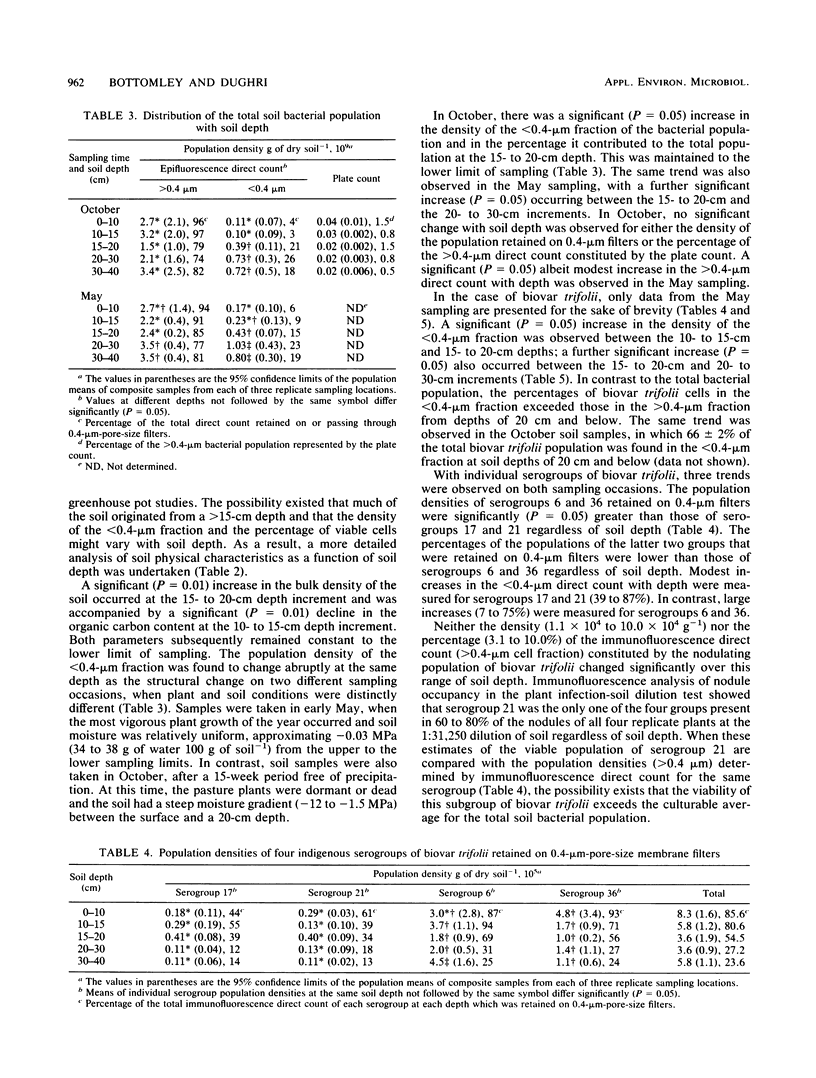
Bacterial cells small enough to pass through 0.4-μm-pore-size filters made up 5 to 9% of the indigenous bacterial population in 0- to 20-cm-depth samples of Abiqua silty clay loam. Within the same soil samples, cells of a similar dimension were stained with fluorescent antibodies specific to each of four antigenically distinct indigenous serogroups of Rhizobium leguminosarum bv. trifolii and made up 22 to 34% of the soil population of the four serogroups. Despite the extensive contribution of small cells to these soil populations, no evidence of their being capable of either growth or nodulation was obtained. The density of soil bacteria which could be cultured ranged between 0.5 and 8.5% of the >0.4-μm direct count regardless of media, season of sampling, or soil depth. In the same soil samples, the viable nodulating populations of biovar trifolii determined by the plant infection soil dilution technique ranged between 1 and 10% of the >0.4-μm direct-immunofluorescence count of biovar trifolii. The <0.4-μm cell populations of both total soil bacteria and biovar trifolii changed abruptly between the 10- to 15-cm and 15- to 20-cm soil depth increments, increasing from 5 to 20% and from 20 to 50%, respectively, of their direct-count totals. The increase in density of the small-cell population corresponded to a significant increase in soil bulk density (1.07 to 1.21 g cm−3). The percent contribution of the <0.4-μm direct count to individual serogroup totals increased with soil depth by approximately 2-fold (39 to 87%) for serogroups 17 and 21 and by 12-fold (6 to 75%) for serogroups 6 and 36.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almendras A. S., Bottomley P. J. Influence of Lime and Phosphate on Nodulation of Soil-Grown Trifolium subterraneum L. by Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1987 Sep;53(9):2090–2097. doi: 10.1128/aem.53.9.2090-2097.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H. C., Casida L. E., Jr Responses of indigenous microorganisms to soil incubation as viewed by transmission electron microscopy of cell thin sections. J Bacteriol. 1973 Mar;113(3):1462–1473. doi: 10.1128/jb.113.3.1462-1473.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae H. C., Cota-Robles E. H., Casida L. E. Microflora of soil as viewed by transmission electron microscopy. Appl Microbiol. 1972 Mar;23(3):637–648. doi: 10.1128/am.23.3.637-648.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken L. R. Separation and purification of bacteria from soil. Appl Environ Microbiol. 1985 Jun;49(6):1482–1487. doi: 10.1128/aem.49.6.1482-1487.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balkwill D. L., Labeda D. P., Casida L. E., Jr Simplified procedures for releasing and concentrating microorganisms from soil for transmission electron microscopy viewing as thin-sectioned and frozen-etched preparations. Can J Microbiol. 1975 Mar;21(3):252–262. doi: 10.1139/m75-036. [DOI] [PubMed] [Google Scholar]
- Casida L. E., Jr Microorganisms in unamended soil as observed by various forms of microscopy and staining. Appl Microbiol. 1971 Jun;21(6):1040–1045. doi: 10.1128/am.21.6.1040-1045.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demezas D. H., Bottomley P. J. Autecology in Rhizospheres and Nodulating Behavior of Indigenous Rhizobium trifolii. Appl Environ Microbiol. 1986 Nov;52(5):1014–1019. doi: 10.1128/aem.52.5.1014-1019.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley M. T., Bohlool B. B. Release of Rhizobium spp. from Tropical Soils and Recovery for Immunofluorescence Enumeration. Appl Environ Microbiol. 1981 Aug;42(2):241–248. doi: 10.1128/aem.42.2.241-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N. A tentative direct microscopic method for counting living marine bacteria. Can J Microbiol. 1979 Mar;25(3):415–420. doi: 10.1139/m79-063. [DOI] [PubMed] [Google Scholar]
- Kogure K., Simidu U., Taga N., Colwell R. R. Correlation of direct viable counts with heterotrophic activity for marine bacteria. Appl Environ Microbiol. 1987 Oct;53(10):2332–2337. doi: 10.1128/aem.53.10.2332-2337.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viteri S. E., Schmidt E. L. Ecology of Indigenous Soil Rhizobia: Response of Bradyrhizobium japonicum to Readily Available Substrates. Appl Environ Microbiol. 1987 Aug;53(8):1872–1875. doi: 10.1128/aem.53.8.1872-1875.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



