Abstract
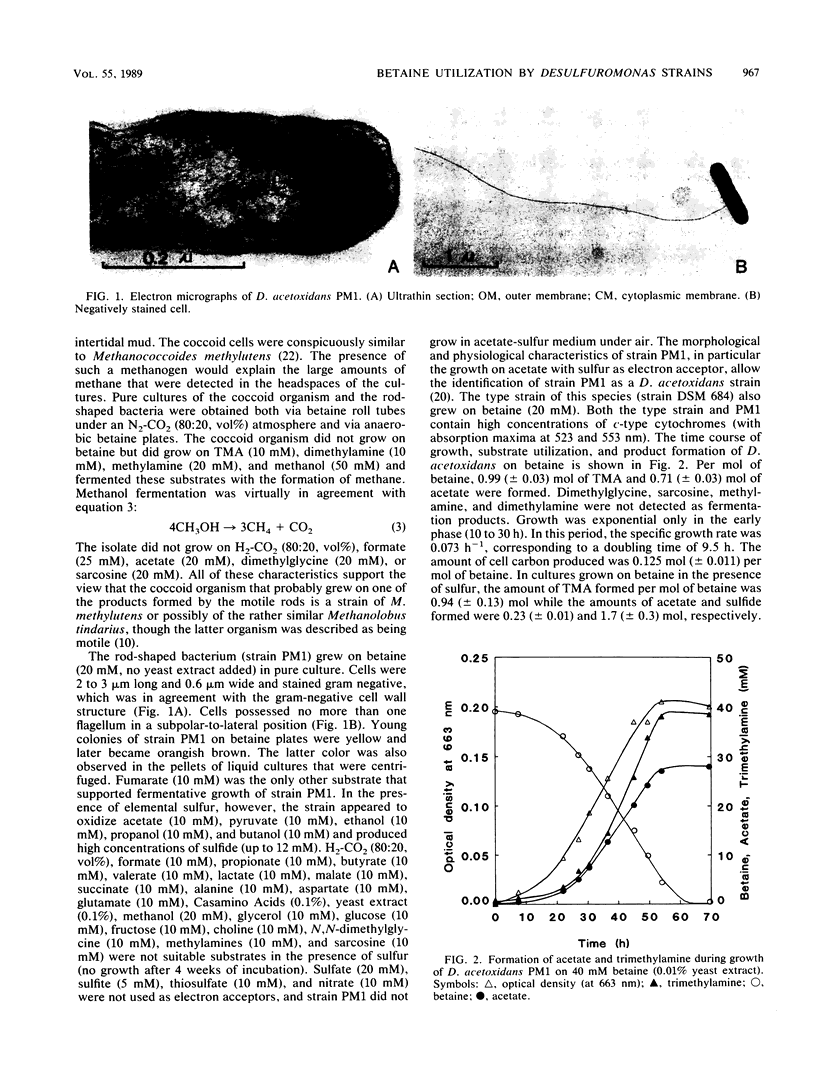
Two bacterial strains were dominant in anaerobic enrichment cultures with betaine (N,N,N-trimethylglycine) as a substrate and intertidal mud as an inoculum. One was a coccoid bacterium which was a trimethylamine (TMA)-fermenting methanogen similar to Methanococcoides methylutens. The other strain, a rod-shaped, gram-negative, motile bacterium, fermented betaine. On the basis of its ability to oxidize acetate and ethanol to CO2 with sulfur as an electron acceptor, its inability to reduce sulfate and sulfite, its morphology, the presence of c-type cytochromes, and other characteristics, the isolated strain PM1 was identified as Desulfuromonas acetoxidans. Although only malate and fumarate were known as substrates for fermentative growth of this species, the type strain (DSM 684) also fermented betaine. Strain PM1 grew with a doubling time of 9.5 h at 30°C on betaine and produced approximately 1 mol of TMA per mol of betaine, 0.75 mol of acetate, and presumably CO2 as fermentation products but only in the presence of selenite (100 nM). In this fermentation, betaine is probably reductively cleaved to TMA and acetate, and part of the acetate is then oxidized to CO2 to provide the reducing equivalents for the initial cleavage reaction. In the presence of sulfur, betaine was converted to TMA and presumably CO2 with the formation of sulfide; then, only traces of acetate were produced.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Franklin M. J., Wiebe W. J., Whitman W. B. Populations of methanogenic bacteria in a georgia salt marsh. Appl Environ Microbiol. 1988 May;54(5):1151–1157. doi: 10.1128/aem.54.5.1151-1157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhoff J. F., Rodriguez-Valera F. Betaine is the main compatible solute of halophilic eubacteria. J Bacteriol. 1984 Oct;160(1):478–479. doi: 10.1128/jb.160.1.478-479.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M. Metabolism of trimethylamine, choline, and glycine betaine by sulfate-reducing and methanogenic bacteria in marine sediments. Appl Environ Microbiol. 1984 Oct;48(4):719–725. doi: 10.1128/aem.48.4.719-725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortstee G. J. The aerobic decomposition of choline by microorganisms. I. The ability of aerobic organisms, particularly coryneform bacteria, to utilize choline as the sole carbon and nitrogen source. Arch Mikrobiol. 1970;71(3):235–244. [PubMed] [Google Scholar]
- Le Rudulier D., Bouillard L. Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol. 1983 Jul;46(1):152–159. doi: 10.1128/aem.46.1.152-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Rudulier D., Strom A. R., Dandekar A. M., Smith L. T., Valentine R. C. Molecular biology of osmoregulation. Science. 1984 Jun 8;224(4653):1064–1068. doi: 10.1126/science.224.4653.1064. [DOI] [PubMed] [Google Scholar]
- Müller E., Fahlbusch K., Walther R., Gottschalk G. Formation of N,N-Dimethylglycine, Acetic Acid, and Butyric Acid from Betaine by Eubacterium limosum. Appl Environ Microbiol. 1981 Sep;42(3):439–445. doi: 10.1128/aem.42.3.439-445.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naumann E., Hippe H., Gottschalk G. Betaine: New Oxidant in the Stickland Reaction and Methanogenesis from Betaine and l-Alanine by a Clostridium sporogenes-Methanosarcina barkeri Coculture. Appl Environ Microbiol. 1983 Feb;45(2):474–483. doi: 10.1128/aem.45.2.474-483.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfennig N., Biebl H. Desulfuromonas acetoxidans gen. nov. and sp. nov., a new anaerobic, sulfur-reducing, acetate-oxidizing bacterium. Arch Microbiol. 1976 Oct 11;110(1):3–12. doi: 10.1007/BF00416962. [DOI] [PubMed] [Google Scholar]
- Smith L. T., Pocard J. A., Bernard T., Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988 Jul;170(7):3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Ferry J. G. Isolation and Characterization of a Methylotrophic Marine Methanogen, Methanococcoides methylutens gen. nov., sp. nov. Appl Environ Microbiol. 1983 Feb;45(2):684–690. doi: 10.1128/aem.45.2.684-690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]