Abstract
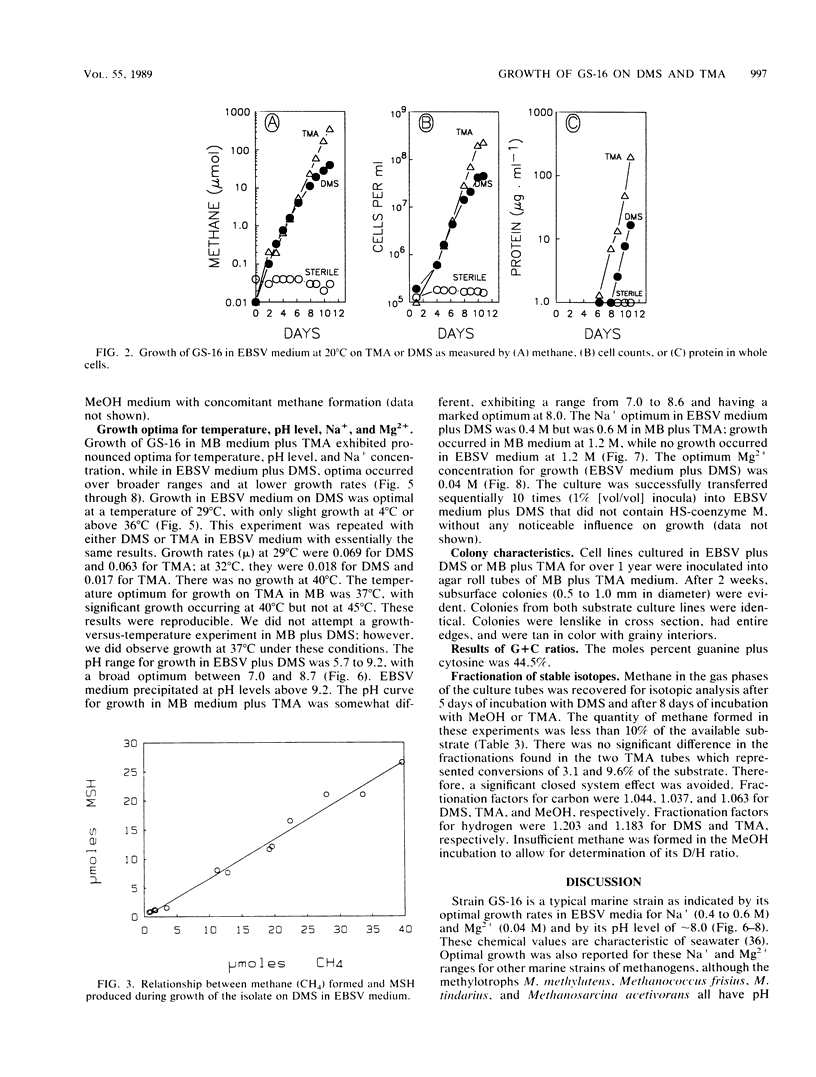
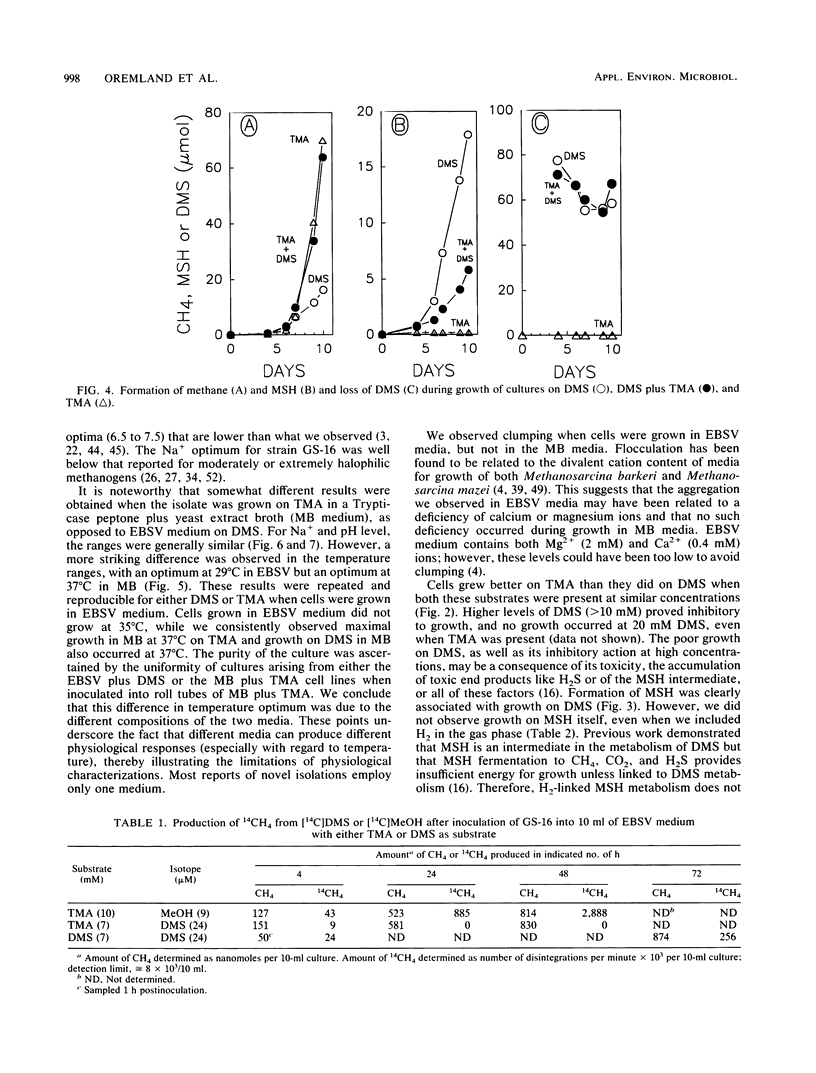
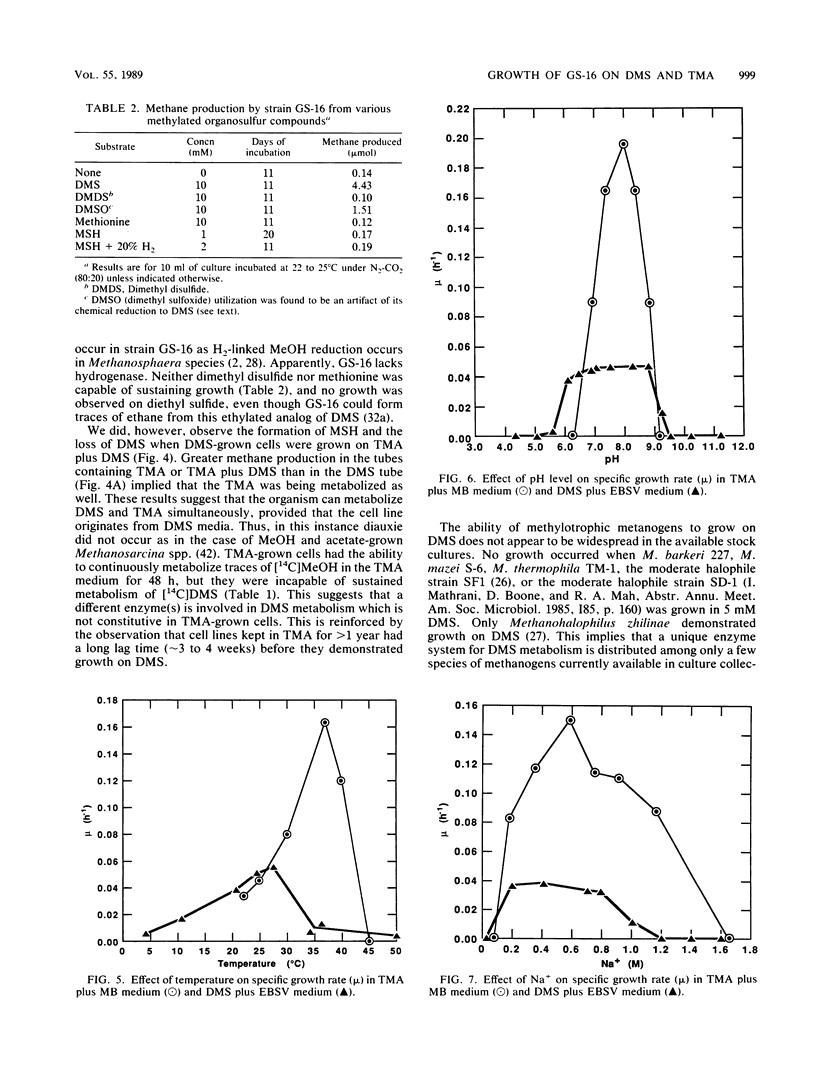
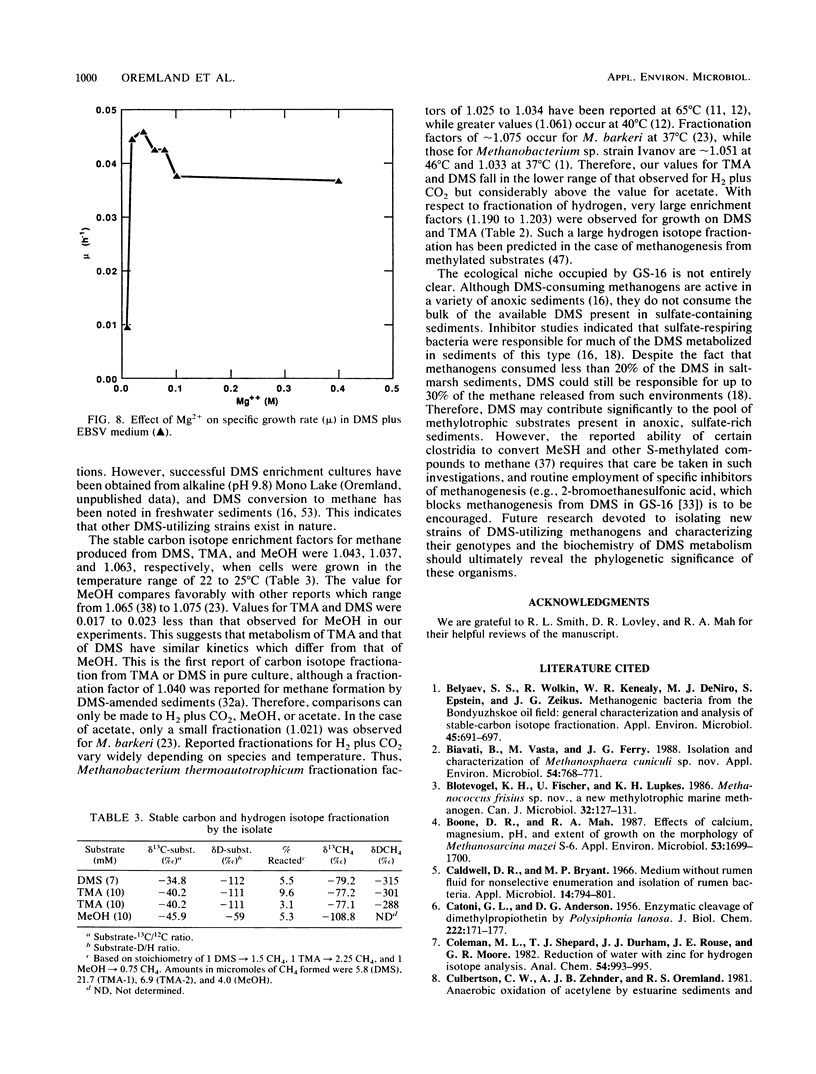
Characteristics of an obligately methylotrophic coccoid methanogen (strain GS-16) previously isolated from estuarine sediment are described. Growth was demonstrated on dimethyl sulfide (DMS) or trimethylamine (TMA), but not on methane thiol, methane thiol plus hydrogen, dimethyl disulfide, or methionine. DMS-grown cells were able to metabolize DMS and TMA simultaneously when inoculated into media containing substrate levels of these compounds. However, TMA-grown cells could not metabolize [14C]DMS to 14CH4, although they could convert [14C]methanol to 14CH4. These results suggest that metabolism of DMS proceeds along a somewhat different route than that of TMA and perhaps also that of methanol. The organism exhibited doubling times of 23 and 32 h for growth (25°C) in mineral media on TMA and DMS, respectively. Doubling times were more rapid (∼6 h) when the organisms were grown on TMA in complex broth. In mineral media, the fastest growth on DMS occurred between pH levels of 7.0 and 8.7, at 29°C, and with 0.2 to 0.4 M Na+ and 0.04 M Mg2+. Somewhat different results occurred for growth on TMA in complex broth. Cells had a moles percent G+C value of 44.5% for their DNA. Growth on DMS, TMA, and methanol yielded stable carbon isotope fractionation factors of 1.044, 1.037, and 1.063, respectively. Fractionation factors for hydrogen were 1.203 (DMS) and 1.183 (TMA).
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON D. G., CANTONI G. L. Enzymatic cleavage of dimethylpropiothetin by Polysiphonia lanosa. J Biol Chem. 1956 Sep;222(1):171–177. [PubMed] [Google Scholar]
- Biavati B., Vasta M., Ferry J. G. Isolation and characterization of "Methanosphaera cuniculi" sp. nov. Appl Environ Microbiol. 1988 Mar;54(3):768–771. doi: 10.1128/aem.54.3.768-771.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone D. R., Mah R. A. Effects of Calcium, Magnesium, pH, and Extent of Growth on the Morphology of Methanosarcina mazei S-6. Appl Environ Microbiol. 1987 Jul;53(7):1699–1700. doi: 10.1128/aem.53.7.1699-1700.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Bryant M. P. Medium without rumen fluid for nonselective enumeration and isolation of rumen bacteria. Appl Microbiol. 1966 Sep;14(5):794–801. doi: 10.1128/am.14.5.794-801.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culbertson C. W., Zehnder A. J., Oremland R. S. Anaerobic oxidation of acetylene by estuarine sediments and enrichment cultures. Appl Environ Microbiol. 1981 Feb;41(2):396–403. doi: 10.1128/aem.41.2.396-403.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin M. J., Wiebe W. J., Whitman W. B. Populations of methanogenic bacteria in a georgia salt marsh. Appl Environ Microbiol. 1988 May;54(5):1151–1157. doi: 10.1128/aem.54.5.1151-1157.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes A. W., King R. E., Unger P. D., Phillips T. D., Hatkin J., Bowen J. H. Aflatoxicosis in swine. J Am Vet Med Assoc. 1978 Jun 1;172(11):1295–1297. [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Oremland R. S., Catena A., Miller L. G., Capone D. G. Metabolism of reduced methylated sulfur compounds in anaerobic sediments and by a pure culture of an estuarine methanogen. Appl Environ Microbiol. 1986 Nov;52(5):1037–1045. doi: 10.1128/aem.52.5.1037-1045.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiene R. P., Visscher P. T. Production and fate of methylated sulfur compounds from methionine and dimethylsulfoniopropionate in anoxic salt marsh sediments. Appl Environ Microbiol. 1987 Oct;53(10):2426–2434. doi: 10.1128/aem.53.10.2426-2434.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M., Klug M. J., Lovley D. R. Metabolism of acetate, methanol, and methylated amines in intertidal sediments of lowes cove, maine. Appl Environ Microbiol. 1983 Jun;45(6):1848–1853. doi: 10.1128/aem.45.6.1848-1853.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King G. M. Metabolism of trimethylamine, choline, and glycine betaine by sulfate-reducing and methanogenic bacteria in marine sediments. Appl Environ Microbiol. 1984 Oct;48(4):719–725. doi: 10.1128/aem.48.4.719-725.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krzycki J. A., Kenealy W. R., Deniro M. J., Zeikus J. G. Stable Carbon Isotope Fractionation by Methanosarcina barkeri during Methanogenesis from Acetate, Methanol, or Carbon Dioxide-Hydrogen. Appl Environ Microbiol. 1987 Oct;53(10):2597–2599. doi: 10.1128/aem.53.10.2597-2599.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathrani I. M., Boone D. R. Isolation and characterization of a moderately halophilic methanogen from a solar saltern. Appl Environ Microbiol. 1985 Jul;50(1):140–143. doi: 10.1128/aem.50.1.140-143.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathrani I. M., Boone D. R., Mah R. A., Fox G. E., Lau P. P. Methanohalophilus zhilinae sp. nov., an alkaliphilic, halophilic, methylotrophic methanogen. Int J Syst Bacteriol. 1988 Apr;38(2):139–142. doi: 10.1099/00207713-38-2-139. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol. 1985 Mar;141(2):116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- Oremland R. S., Polcin S. Methanogenesis and sulfate reduction: competitive and noncompetitive substrates in estuarine sediments. Appl Environ Microbiol. 1982 Dec;44(6):1270–1276. doi: 10.1128/aem.44.6.1270-1276.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oremland R. S., Zehr J. P. Formation of methane and carbon dioxide from dimethylselenide in anoxic sediments and by a methanogenic bacterium. Appl Environ Microbiol. 1986 Nov;52(5):1031–1036. doi: 10.1128/aem.52.5.1031-1036.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterek J. R., Smith P. H. Isolation and characterization of a halophilic methanogen from great salt lake. Appl Environ Microbiol. 1985 Oct;50(4):877–881. doi: 10.1128/aem.50.4.877-881.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston J. F., Boone D. R. Analytical determination of the buoyant density of DNA in acrylamide gels after preparative CsCl gradient centrifugation. FEBS Lett. 1973 Dec 1;37(2):321–324. doi: 10.1016/0014-5793(73)80487-9. [DOI] [PubMed] [Google Scholar]
- ROSENFELD W. D., SILVERMAN S. R. Carbon isotope fractionation in bacterial production of methane. Science. 1959 Dec 11;130(3389):1658–1659. doi: 10.1126/science.130.3389.1658-a. [DOI] [PubMed] [Google Scholar]
- Rimbault A., Niel P., Virelizier H., Darbord J. C., Leluan G. l-Methionine, a Precursor of Trace Methane in Some Proteolytic Clostridia. Appl Environ Microbiol. 1988 Jun;54(6):1581–1586. doi: 10.1128/aem.54.6.1581-1586.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Smith M. R., Mah R. A. Growth and methanogenesis by Methanosarcina strain 227 on acetate and methanol. Appl Environ Microbiol. 1978 Dec;36(6):870–879. doi: 10.1128/aem.36.6.870-879.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Baron S. F., Ferry J. G. Methanosarcina acetivorans sp. nov., an Acetotrophic Methane-Producing Bacterium Isolated from Marine Sediments. Appl Environ Microbiol. 1984 May;47(5):971–978. doi: 10.1128/aem.47.5.971-978.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowers K. R., Ferry J. G. Isolation and Characterization of a Methylotrophic Marine Methanogen, Methanococcoides methylutens gen. nov., sp. nov. Appl Environ Microbiol. 1983 Feb;45(2):684–690. doi: 10.1128/aem.45.2.684-690.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehnder A. J., Huser B. A., Brock T. D., Wuhrmann K. Characterization of an acetate-decarboxylating, non-hydrogen-oxidizing methane bacterium. Arch Microbiol. 1980 Jan;124(1):1–11. doi: 10.1007/BF00407022. [DOI] [PubMed] [Google Scholar]
- Zhilina T. N. Tonkoe stroenie metanosartsiny. Mikrobiologiia. 1971 Jul-Aug;40(4):674–680. [PubMed] [Google Scholar]
- Zinder S. H., Mah R. A. Isolation and Characterization of a Thermophilic Strain of Methanosarcina Unable to Use H(2)-CO(2) for Methanogenesis. Appl Environ Microbiol. 1979 Nov;38(5):996–1008. doi: 10.1128/aem.38.5.996-1008.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]



