Abstract
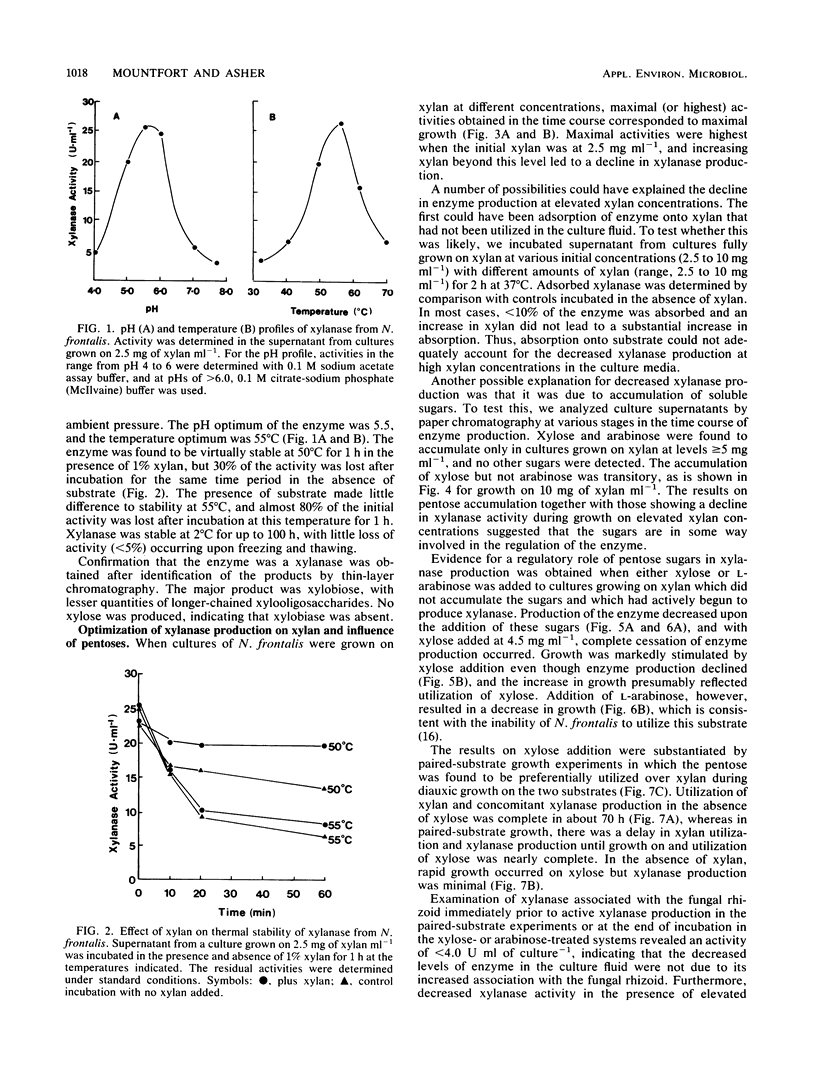
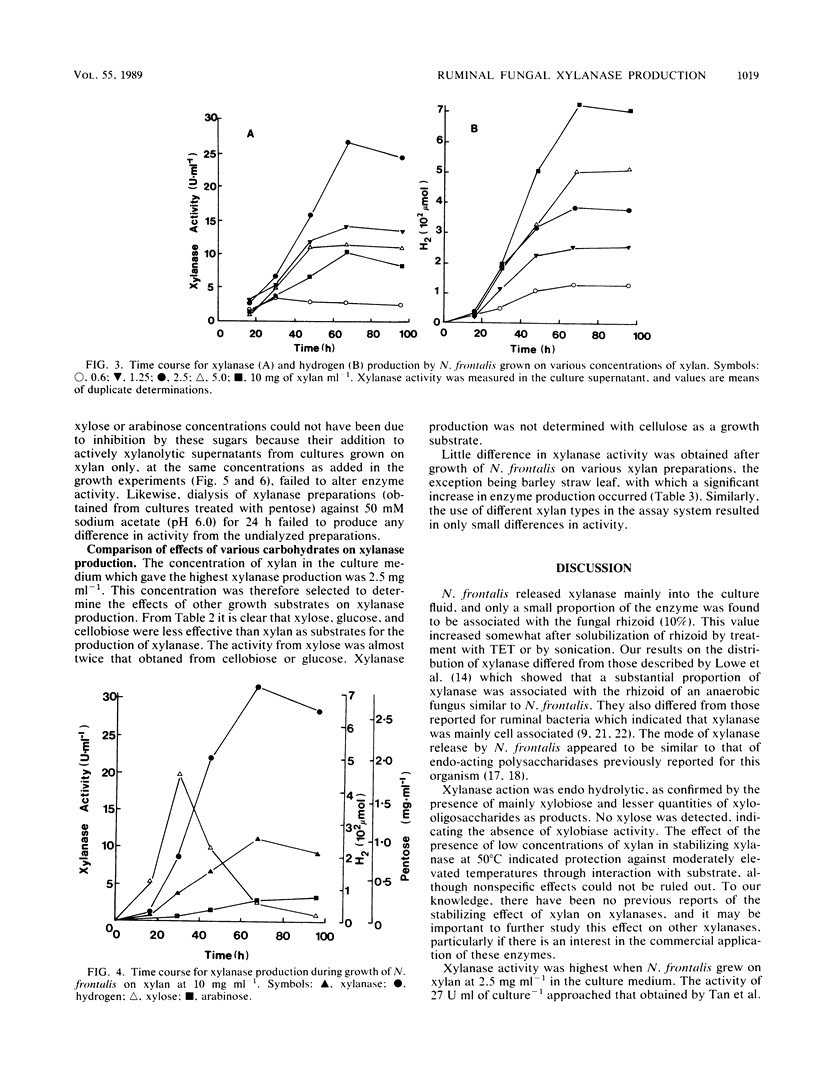
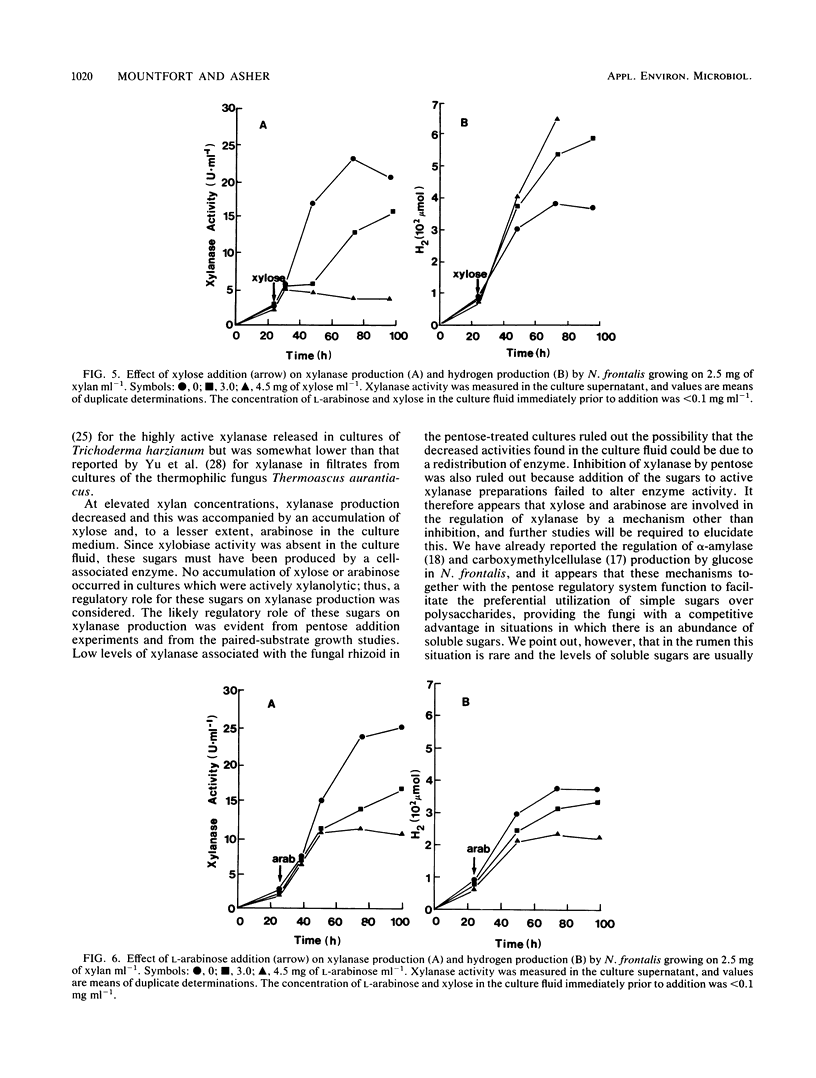
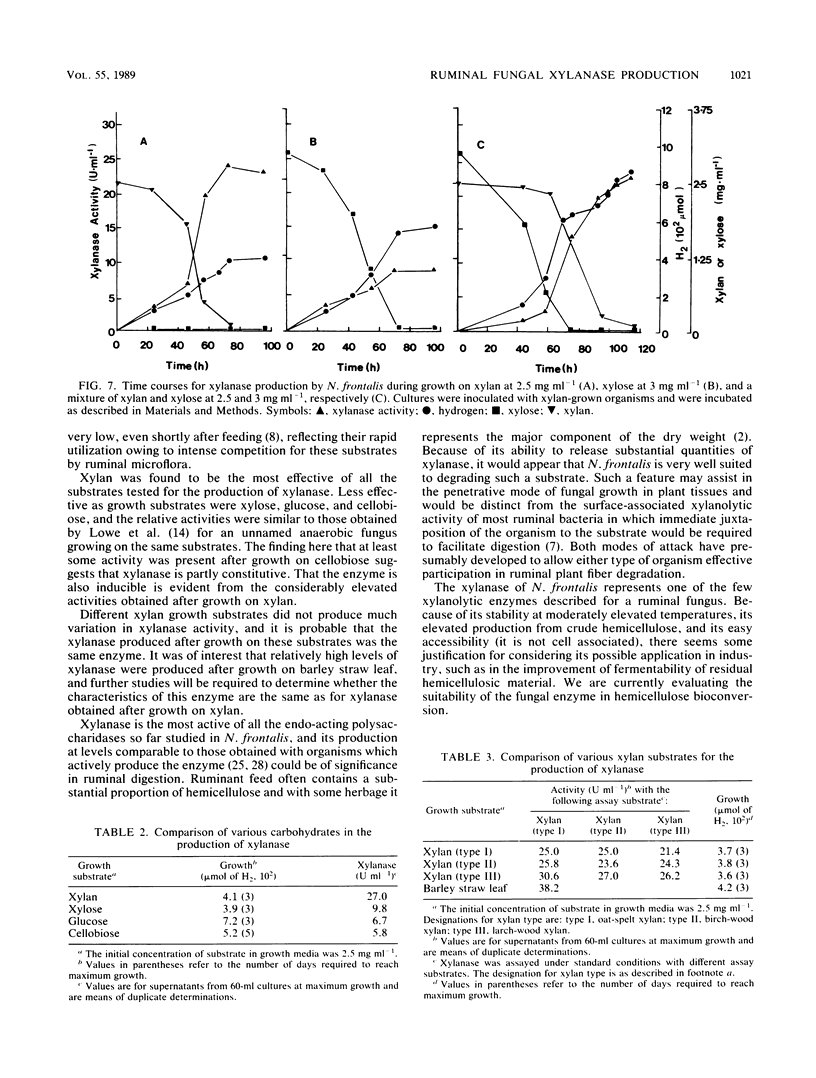
Xylanase (1,4-beta-D-xylan xylanohydrolase, EC 3.2.1.8) production was investigated in the ruminal anaerobic fungus Neocallimastix frontalis. The enzyme was released principally into the culture fluid and had pH and temperature optima of 5.5 and 55 degrees C, respectively. In the presence of low concentrations of substrate, the enzyme was stabilized at 50 degrees C. Xylobiose was the principal product of xylanase action, with lesser amounts of longer-chained xylooligosaccharides. No xylose was detected, indicating that xylobiase activity was absent. Activities of xylanase up to 27 U ml-1 (1 U represents 1 micromol of xylose equivalents released min-1) were obtained for cultures grown on xylan (from oat spelt) at 2.5 mg ml-1 in shaken cultures. No growth occurred in unshaken cultures. Xylanase production declined with elevated concentrations of xylan (less than 2.5 mg ml-1), and this was accompanied by an accumulation of xylose and, to a lesser extent, arabinose. Addition of either pentose to cultures grown on low levels of xylan in which neither sugar accumulated suppressed xylanase production, and in growth studies with the paired substrates xylan-xylose, active production of the enzyme occurred during growth on xylan only after xylose had been preferentially utilized. When cellobiose, glucose, and xylose were tested as growth substrates for the production of xylanase (each initially at 2.5 mg ml-1), they were found to be less effective than xylan, and use of xylan from different origins (birch wood or larch wood) as the growth substrate or in the assay system resulted in only marginal differences in enzyme activity. However, elevated production of xylanase occurred during growth on crude hemicellulose (barley straw leaf). The results are discussed in relation to the role of the anaerobic fungi in the ruminal ecosystem, and the possible application of the enzyme in bioconversion processes is also considered.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAILEY R. W. The reaction of pentoses with anthrone. Biochem J. 1958 Apr;68(4):669–672. doi: 10.1042/bj0680669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch W. E., Wolfe R. S. New approach to the cultivation of methanogenic bacteria: 2-mercaptoethanesulfonic acid (HS-CoM)-dependent growth of Methanobacterium ruminantium in a pressureized atmosphere. Appl Environ Microbiol. 1976 Dec;32(6):781–791. doi: 10.1128/aem.32.6.781-791.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T., Mountfort D. O. Cellulose fermentation by a rumen anaerobic fungus in both the absence and the presence of rumen methanogens. Appl Environ Microbiol. 1981 Dec;42(6):1103–1110. doi: 10.1128/aem.42.6.1103-1110.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. Rumen anaerobic fungi of cattle and sheep. Appl Environ Microbiol. 1979 Jul;38(1):148–158. doi: 10.1128/aem.38.1.148-158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauchop T. The rumen anaerobic fungi: colonizers of plant fibre. Ann Rech Vet. 1979;10(2-3):246–248. [PubMed] [Google Scholar]
- Clarke R. T., Bailey R. W., Gaillard B. D. Growth of rumen bacteria on plant cell wall polysaccharides. J Gen Microbiol. 1969 Apr;56(1):9–86. [PubMed] [Google Scholar]
- De Mot R., Verachtert H. Purification and Characterization of Extracellular Amylolytic Enzymes from the Yeast Filobasidium capsuligenum. Appl Environ Microbiol. 1985 Dec;50(6):1474–1482. doi: 10.1128/aem.50.6.1474-1482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehority B. A. Mechanism of isolated hemicellulose and xylan degradation by cellulolytic rumen bacteria. Appl Microbiol. 1968 May;16(5):781–786. doi: 10.1128/am.16.5.781-786.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe S. E., Theodorou M. K., Trinci A. P. Cellulases and xylanase of an anaerobic rumen fungus grown on wheat straw, wheat straw holocellulose, cellulose, and xylan. Appl Environ Microbiol. 1987 Jun;53(6):1216–1223. doi: 10.1128/aem.53.6.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production and regulation of cellulase by two strains of the rumen anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1985 May;49(5):1314–1322. doi: 10.1128/aem.49.5.1314-1322.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Production of alpha-Amylase by the Ruminal Anaerobic Fungus Neocallimastix frontalis. Appl Environ Microbiol. 1988 Sep;54(9):2293–2299. doi: 10.1128/aem.54.9.2293-2299.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountfort D. O., Asher R. A. Role of catabolite regulatory mechanisms in control of carbohydrate utilization by the rumen anaerobic fungus Neocallimastix frontalis. Appl Environ Microbiol. 1983 Dec;46(6):1331–1338. doi: 10.1128/aem.46.6.1331-1338.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce P. D., Bauchop T. Glycosidases of the rumen anaerobic fungus Neocallimastix frontalis grown on cellulosic substrates. Appl Environ Microbiol. 1985 May;49(5):1265–1269. doi: 10.1128/aem.49.5.1265-1269.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp P., Wagner F. Production and Properties of Xylan-Degrading Enzymes from Cellulomonas uda. Appl Environ Microbiol. 1986 Apr;51(4):746–752. doi: 10.1128/aem.51.4.746-752.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Williams A. G., Orpin C. G. Polysaccharide-degrading enzymes formed by three species of anaerobic rumen fungi grown on a range of carbohydrate substrates. Can J Microbiol. 1987 May;33(5):418–426. doi: 10.1139/m87-071. [DOI] [PubMed] [Google Scholar]



