Abstract
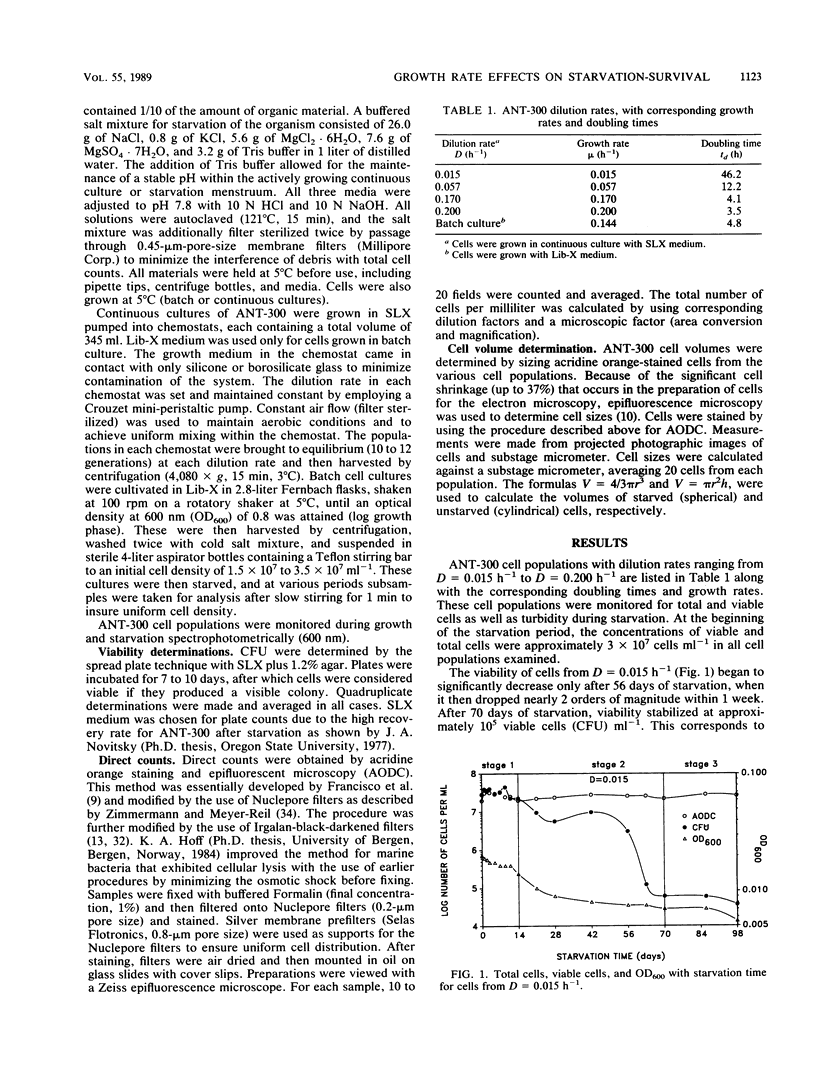
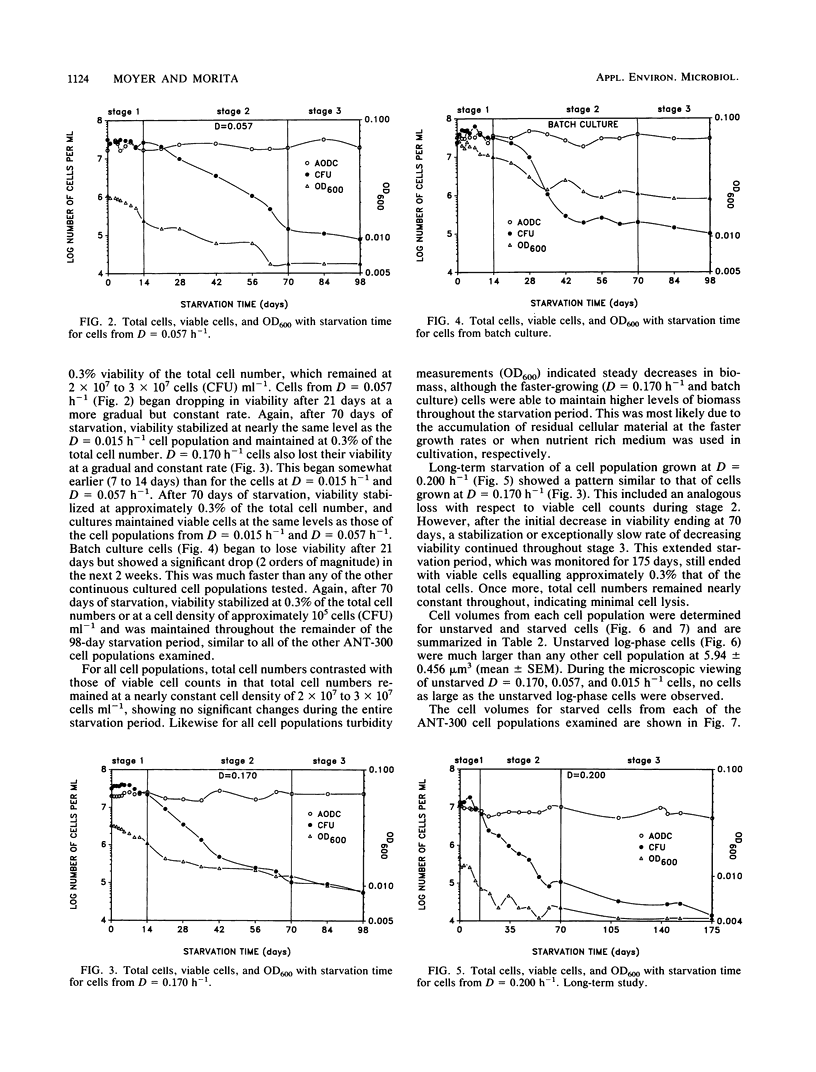
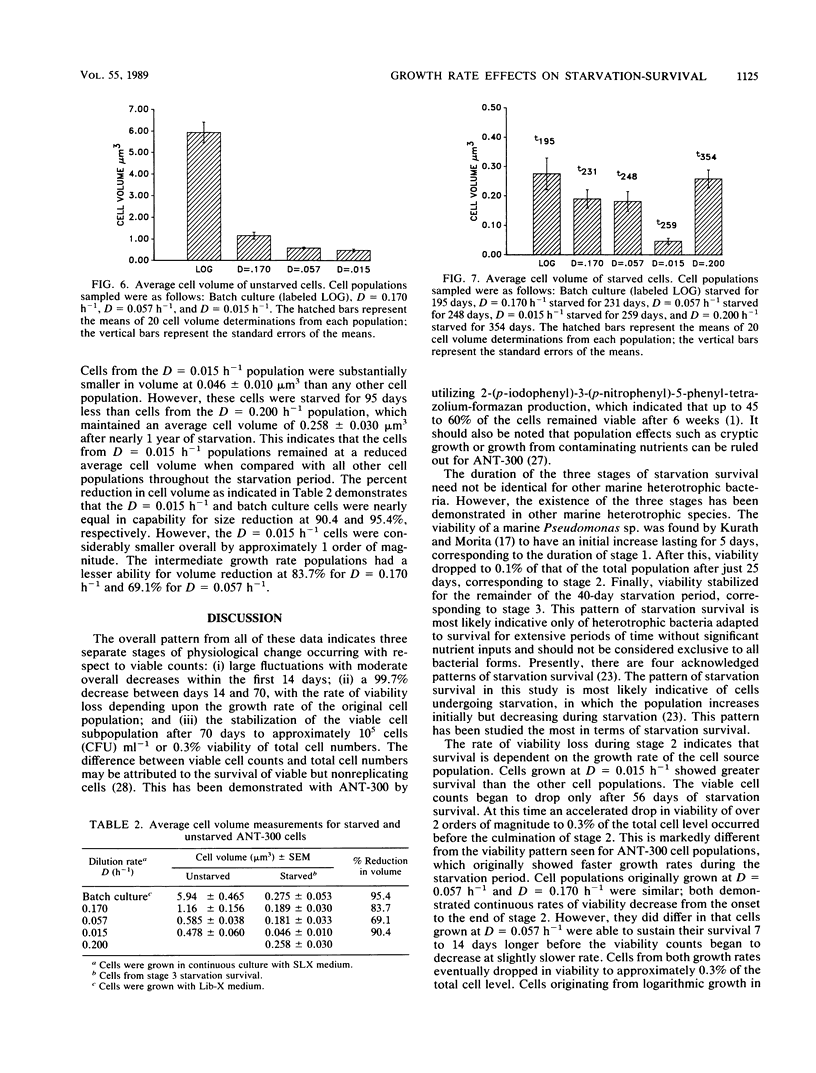
Cell populations of the marine bacterium ANT-300, from either batch or continuous culture with dilution rates ranging from D = 0.015 h−1 to D = 0.200 h−1, were monitored for viability, direct counts, and optical density for 98 days under starvation conditions. Three stages of starvation survival were observed for each of the cell populations. Although direct counts remained at 2 × 107 to 3 × 107 cells ml−1 throughout the starvation period, large fluctuations occurred in cell viability during stage 1 (0 to 14 days) of starvation survival. Stage 2 (14 to 70 days) involved an overall decrease in viability for each of the cell populations; the rate of viability loss was dependent upon the growth rate. Cell viability stabilized at approximately 0.3% of the direct count in stage 3 (70 to 98 days). Long-term starvation corresponded to the prolongation of stage 3 starvation survival. Cell volumes for each of the cell populations decreased with the length of the starvation period. However, the cell volume of starved cells was also dependent more on growth rate than on the length of the time starved. We hypothesize that the cell population with the slowest growth rate is most closely representative of cells found in the oligotrophic marine environment.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amy P. S., Pauling C., Morita R. Y. Starvation-survival processes of a marine Vibrio. Appl Environ Microbiol. 1983 Mar;45(3):1041–1048. doi: 10.1128/aem.45.3.1041-1048.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R. T. Dissolved organic carbon from deep waters resists microbial oxidation. Nature. 1968 Oct 19;220(5164):274–275. doi: 10.1038/220274a0. [DOI] [PubMed] [Google Scholar]
- Francisco D. E., Mah R. A., Rabin A. C. Acridine orange-epifluorescence technique for counting bacteria in natural waters. Trans Am Microsc Soc. 1973 Jul;92(3):416–421. [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannasch H. W. Estimations of bacterial growth rates in natural waters. J Bacteriol. 1969 Jul;99(1):156–160. doi: 10.1128/jb.99.1.156-160.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurath G., Morita R. Y. Starvation-Survival Physiological Studies of a Marine Pseudomonas sp. Appl Environ Microbiol. 1983 Apr;45(4):1206–1211. doi: 10.1128/aem.45.4.1206-1211.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita R. Y. Microbial life in the deep sea. Can J Microbiol. 1980 Dec;26(12):1375–1385. doi: 10.1139/m80-230. [DOI] [PubMed] [Google Scholar]
- Roszak D. B., Colwell R. R. Survival strategies of bacteria in the natural environment. Microbiol Rev. 1987 Sep;51(3):365–379. doi: 10.1128/mr.51.3.365-379.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smits J. D., Riemann B. Calculation of cell production from [h]thymidine incorporation with freshwater bacteria. Appl Environ Microbiol. 1988 Sep;54(9):2213–2219. doi: 10.1128/aem.54.9.2213-2219.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tranvik L. J., Höfle M. G. Bacterial growth in mixed cultures on dissolved organic carbon from humic and clear waters. Appl Environ Microbiol. 1987 Mar;53(3):482–488. doi: 10.1128/aem.53.3.482-488.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Novitsky T. J., Quinby H. L., Valois F. W. Determination of bacterial number and biomass in the marine environment. Appl Environ Microbiol. 1977 Apr;33(4):940–946. doi: 10.1128/aem.33.4.940-946.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]



