Abstract
A highly polymorphic Xenorhabdus luminescens strain was isolated. The primary form of X. luminescens was luminescent and nonswarming and produced a yellow pigment and antimicrobial substances. The primary form generated a secondary form that had a distinct orange pigmentation, was weakly luminescent, and did not produce antimicrobial substances. Both the primary and secondary forms generated a set of colony variants at frequencies that exceeded normal rates for spontaneous mutation. The variant forms include nonswarming and swarming forms that formed large colonies and a small-colony (SC) form. The primary and secondary forms generated their SC forms at frequencies of between 1 and 14% and 1 and 2%, respectively. The SC forms were distinct from their parental primary and secondary forms in colony and cellular morphology and in protein composition. The cellular morphology and protein patterns of the nonswarming and swarming colony variants were all very similar. The DNA fingerprints of all forms were similar. Each SC-form colony reverted at high frequency to the form from which it was derived. The proportion of parental-type cells in the SC-form colonies varied with age, with young colonies containing as few as 0.0002% parental-type cells. The primary-to-secondary switch was stable, but all the other colony forms were able to switch at high frequencies to the alternative colony phenotypes.
Full text
PDF



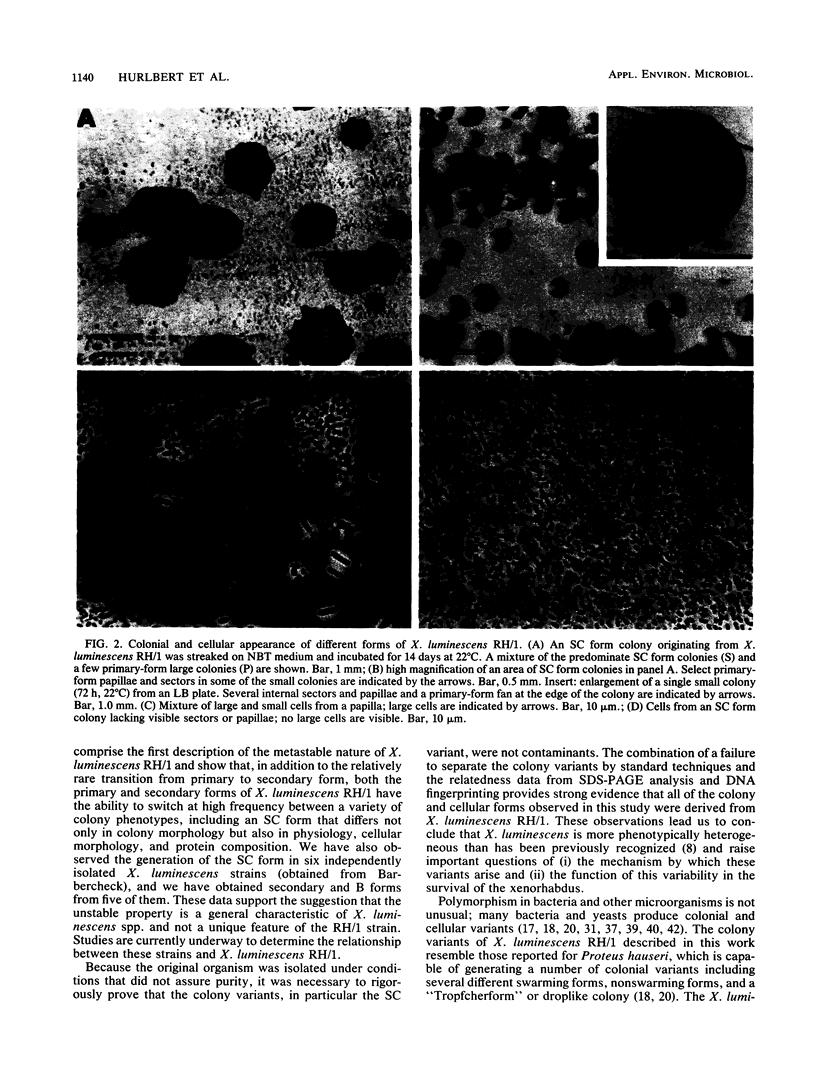



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. J Gen Microbiol. 1982 Dec;128(12):3061–3065. doi: 10.1099/00221287-128-12-3061. [DOI] [PubMed] [Google Scholar]
- Akhurst R. J., Boemare N. E. A numerical taxonomic study of the genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of X. nematophilus to species. J Gen Microbiol. 1988 Jul;134(7):1835–1845. doi: 10.1099/00221287-134-7-1835. [DOI] [PubMed] [Google Scholar]
- Anderson J. M., Soll D. R. Unique phenotype of opaque cells in the white-opaque transition of Candida albicans. J Bacteriol. 1987 Dec;169(12):5579–5588. doi: 10.1128/jb.169.12.5579-5588.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchholz W. G., Thomashow M. F. Comparison of T-DNA oncogene complements of Agrobacterium tumefaciens tumor-inducing plasmids with limited and wide host ranges. J Bacteriol. 1984 Oct;160(1):319–326. doi: 10.1128/jb.160.1.319-326.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COETZEE J. N., SACKS T. G. Morphological variants of Proteus hauseri. J Gen Microbiol. 1960 Oct;23:209–216. doi: 10.1099/00221287-23-2-209. [DOI] [PubMed] [Google Scholar]
- Claassen P. A., Kortstee G. J., Oosterveld-van Vliet W. M., van Neerven A. R. Colonial heterogeneity of Thiobacillus versutus. J Bacteriol. 1986 Nov;168(2):791–794. doi: 10.1128/jb.168.2.791-794.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb D. I. GABAergic neurons. Sci Am. 1988 Feb;258(2):82–89. doi: 10.1038/scientificamerican0288-82. [DOI] [PubMed] [Google Scholar]
- HOENIGER J. F. CELLULAR CHANGES ACCOMPANYING THE SWARMING OF PROTEUS MIRABILIS. I. OBSERVATIONS OF LIVING CULTURES. Can J Microbiol. 1964 Feb;10:1–9. doi: 10.1139/m64-001. [DOI] [PubMed] [Google Scholar]
- Kramer G. F., Baker J. C., Ames B. N. Near-UV stress in Salmonella typhimurium: 4-thiouridine in tRNA, ppGpp, and ApppGpp as components of an adaptive response. J Bacteriol. 1988 May;170(5):2344–2351. doi: 10.1128/jb.170.5.2344-2351.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McClelland M., Jones R., Patel Y., Nelson M. Restriction endonucleases for pulsed field mapping of bacterial genomes. Nucleic Acids Res. 1987 Aug 11;15(15):5985–6005. doi: 10.1093/nar/15.15.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruchuri D. K., Harshey R. M. Flagellar variation in Serratia marcescens is associated with color variation. J Bacteriol. 1987 Jan;169(1):61–65. doi: 10.1128/jb.169.1.61-65.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poinar G. O., Jr, Thomas G. M. Significance of Achromobacter nematophilus Poinar and Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae). Parasitology. 1966 May;56(2):385–390. doi: 10.1017/s0031182000070980. [DOI] [PubMed] [Google Scholar]
- Schrader J. A., Holmes D. S. Phenotypic switching of Thiobacillus ferrooxidans. J Bacteriol. 1988 Sep;170(9):3915–3923. doi: 10.1128/jb.170.9.3915-3923.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slutsky B., Staebell M., Anderson J., Risen L., Pfaller M., Soll D. R. "White-opaque transition": a second high-frequency switching system in Candida albicans. J Bacteriol. 1987 Jan;169(1):189–197. doi: 10.1128/jb.169.1.189-197.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stray J. E., Klowden M. J., Hurlbert R. E. Toxicity of Bacillus sphaericus crystal toxin to adult mosquitoes. Appl Environ Microbiol. 1988 Sep;54(9):2320–2321. doi: 10.1128/aem.54.9.2320-2321.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sylvester-Bradley R., Thornton P., Jones P. Colony dimorphism in bradyrhizobium strains. Appl Environ Microbiol. 1988 Apr;54(4):1033–1038. doi: 10.1128/aem.54.4.1033-1038.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams F. D., Schwarzhoff R. H. Nature of the swarming phenomenon in Proteus. Annu Rev Microbiol. 1978;32:101–122. doi: 10.1146/annurev.mi.32.100178.000533. [DOI] [PubMed] [Google Scholar]







