Abstract
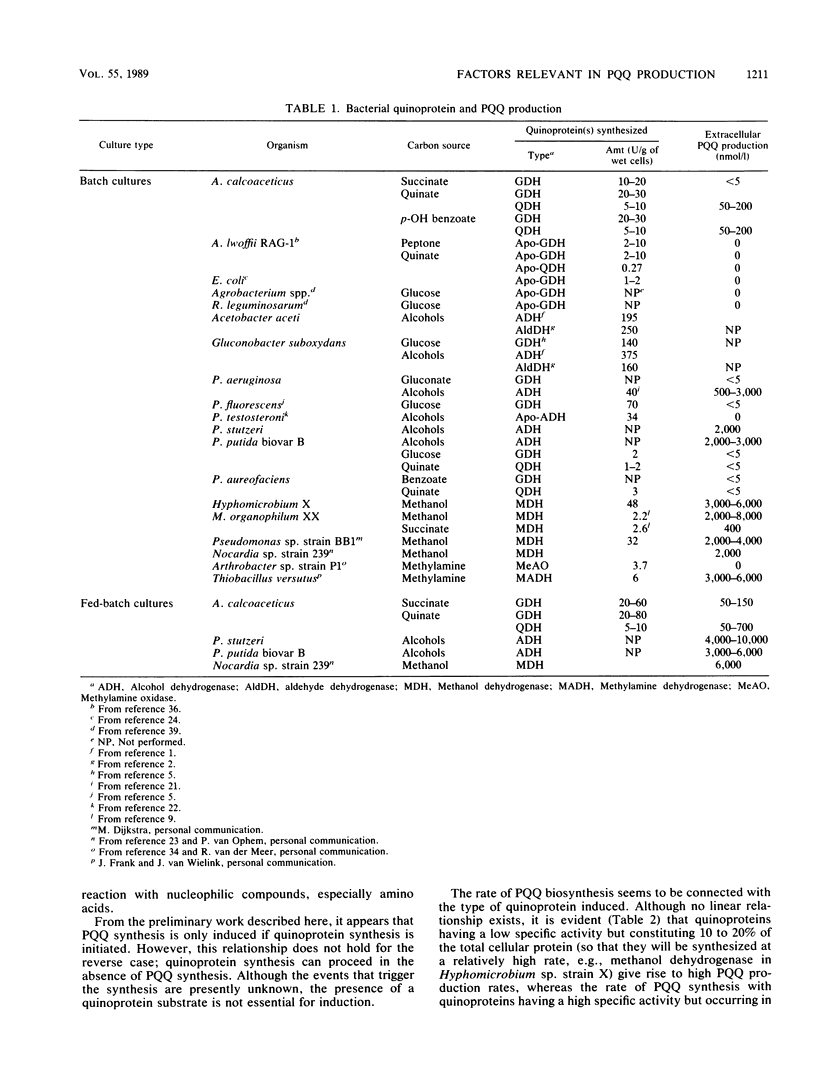
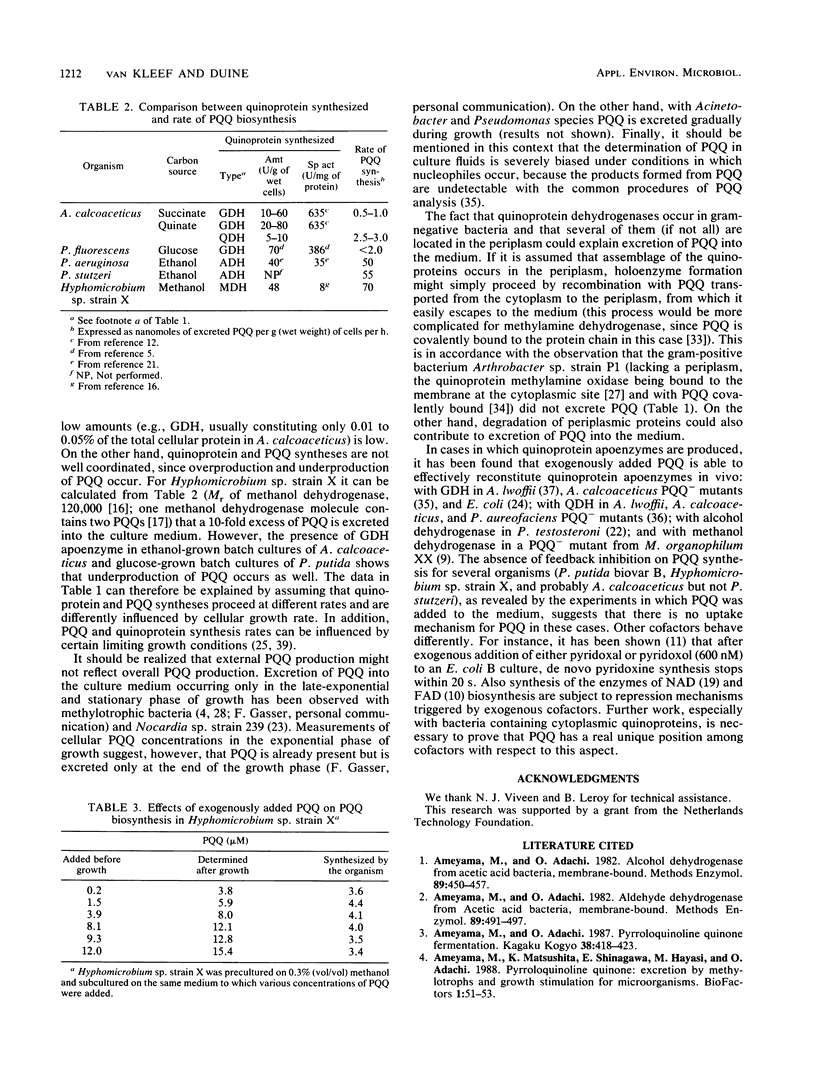
Quinoprotein content and levels of external pyrroloquinoline quinone (PQQ) were determined for several bacteria under a variety of growth conditions. From these data and those from the literature, a number of factors can be indicated which are relevant for PQQ production. Synthesis of PQQ is only started if synthesis of a quinoprotein occurs, but quinoprotein synthesis does not depend on PQQ synthesis. The presence of quinoprotein substrates is not necessary for quinoprotein and PQQ syntheses. Although the extent of PQQ production was determined by the type of organism and quinoprotein produced, coordination between quinoprotein and PQQ syntheses is loose, since underproduction and overproduction of PQQ with respect to quinoprotein were observed. The results can be interpreted to indicate that quinoprotein synthesis depends on the growth rate whereas PQQ synthesis does not. In that view, the highest PQQ production can be achieved under limiting growth conditions, as was shown indeed by the much higher levels of PQQ produced in fed-batch cultures compared with those produced in batch experiments. The presence of nucleophiles, especially amino acids, in culture media may cause losses of PQQ due to transformation into biologically inactive compounds. Some organisms continued to synthesize PQQ de novo when this cofactor was administered exogenously. Most probably PQQ cannot be taken up by either passive diffusion or active transport mechanisms and is therefore not able to exert feedback regulation on its biosynthesis in these organisms.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ameyama M., Matsushita K., Shinagawa E., Hayashi M., Adachi O. Pyrroloquinoline quinone: excretion by methylotrophs and growth stimulation for microorganisms. Biofactors. 1988 Jan;1(1):51–53. [PubMed] [Google Scholar]
- DEMPSEY W. B. CONTROL OF PYRIDOXINE BIOSYNTHESIS IN ESCHERICHIA COLI. J Bacteriol. 1965 Aug;90:431–437. doi: 10.1128/jb.90.2.431-437.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkstra M., Frank J., Jr, van Wielink J. E., Duine J. A. The soluble cytochromes c of methanol-grown Hyphomicrobium X. Evidence against the involvement of autoreduction in electron-acceptor functioning of cytochrome cL. Biochem J. 1988 Apr 15;251(2):467–474. doi: 10.1042/bj2510467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dokter P., Frank J., Duine J. A. Purification and characterization of quinoprotein glucose dehydrogenase from Acinetobacter calcoaceticus L.M.D. 79.41. Biochem J. 1986 Oct 1;239(1):163–167. doi: 10.1042/bj2390163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Jr The prosthetic group of methanol dehydrogenase. Purification and some of its properties. Biochem J. 1980 Apr 1;187(1):221–226. doi: 10.1042/bj1870221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duine J. A., Frank J., Westerling J. Purification and properties of methanol dehydrogenase from Hyphomicrobium x. Biochim Biophys Acta. 1978 Jun 9;524(2):277–287. doi: 10.1016/0005-2744(78)90164-x. [DOI] [PubMed] [Google Scholar]
- Duine J. A., Frank J., van Zeeland J. K. Glucose dehydrogenase from Acinetobacter calcoaceticus: a 'quinoprotein'. FEBS Lett. 1979 Dec 15;108(2):443–446. doi: 10.1016/0014-5793(79)80584-0. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Moat A. G. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev. 1980 Mar;44(1):83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goosen N., Vermaas D. A., van de Putte P. Cloning of the genes involved in synthesis of coenzyme pyrrolo-quinoline-quinone from Acinetobacter calcoaceticus. J Bacteriol. 1987 Jan;169(1):303–307. doi: 10.1128/jb.169.1.303-307.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen B. W., van Kleef M. A., Duine J. A. Quinohaemoprotein alcohol dehydrogenase apoenzyme from Pseudomonas testosteroni. Biochem J. 1986 Mar 15;234(3):611–615. doi: 10.1042/bj2340611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen B., Frank J., Jr, Duine J. A. Quinoprotein alcohol dehydrogenase from ethanol-grown Pseudomonas aeruginosa. Biochem J. 1984 Nov 1;223(3):921–924. doi: 10.1042/bj2230921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommes R. W., van Hell B., Postma P. W., Neijssel O. M., Tempest D. W. The functional significance of glucose dehydrogenase in Klebsiella aerogenes. Arch Microbiol. 1985 Nov;143(2):163–168. doi: 10.1007/BF00411042. [DOI] [PubMed] [Google Scholar]
- Levering P. R., van Dijken J. P., Veenhius M., Harder W. Arthrobacter P1, a fast growing versatile methylotroph with amine oxidase as a key enzyme in the metabolism of methylated amines. Arch Microbiol. 1981 Mar;129(1):72–80. doi: 10.1007/BF00417184. [DOI] [PubMed] [Google Scholar]
- McIntire W. S., Weyler W. Factors affecting the production of pyrroloquinoline quinone by the methylotrophic bacterium W3A1. Appl Environ Microbiol. 1987 Sep;53(9):2183–2188. doi: 10.1128/aem.53.9.2183-2188.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salcher O., Lingens F. Isolation and characterization of a mutant of Pseudomonas aureofaciens ATCC 15926 with an increased capacity for synthesis of pyrrolnitrin. J Gen Microbiol. 1980 Jun;118(2):509–513. doi: 10.1099/00221287-118-2-509. [DOI] [PubMed] [Google Scholar]
- Tresguerres M. E., de Torrontegui G., Ingledew W. M., Cánovas J. L. Regulation of the enzymes of the beta-ketoadipate pathway in Moraxella. Control of quinate oxidation by protocatechuate. Eur J Biochem. 1970 Jul;14(3):445–450. doi: 10.1111/j.1432-1033.1970.tb00309.x. [DOI] [PubMed] [Google Scholar]
- Verwiel P. E., Frank J., Verwiel E. J. Characterization of the second prosthetic group in methanol dehydrogenase from hyphomicrobium X. Eur J Biochem. 1981 Aug;118(2):395–399. doi: 10.1111/j.1432-1033.1981.tb06415.x. [DOI] [PubMed] [Google Scholar]
- van Iersel J., van der Meer R. A., Duine J. A. Methylamine oxidase from Arthrobacter P1. A bacterial copper-quinoprotein amine oxidase. Eur J Biochem. 1986 Dec 1;161(2):415–419. doi: 10.1111/j.1432-1033.1986.tb10461.x. [DOI] [PubMed] [Google Scholar]
- van Kleef M. A., Dokter P., Mulder A. C., Duine J. A. Detection of the cofactor pyrroloquinoline quinone. Anal Biochem. 1987 Apr;162(1):143–149. doi: 10.1016/0003-2697(87)90019-4. [DOI] [PubMed] [Google Scholar]
- van Kleef M. A., Duine J. A. Bacterial NAD(P)-independent quinate dehydrogenase is a quinoprotein. Arch Microbiol. 1988 May;150(1):32–36. doi: 10.1007/BF00409714. [DOI] [PubMed] [Google Scholar]
- van Schie B. J., Hellingwerf K. J., van Dijken J. P., Elferink M. G., van Dijl J. M., Kuenen J. G., Konings W. N. Energy transduction by electron transfer via a pyrrolo-quinoline quinone-dependent glucose dehydrogenase in Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter calcoaceticus (var. lwoffi). J Bacteriol. 1985 Aug;163(2):493–499. doi: 10.1128/jb.163.2.493-499.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer R. A., Jongejan J. A., Duine J. A. Phenylhydrazine as probe for cofactor identification in amine oxidoreductases. Evidence for PQQ as the cofactor in methylamine dehydrogenase. FEBS Lett. 1987 Sep 14;221(2):299–304. doi: 10.1016/0014-5793(87)80944-4. [DOI] [PubMed] [Google Scholar]


