Abstract
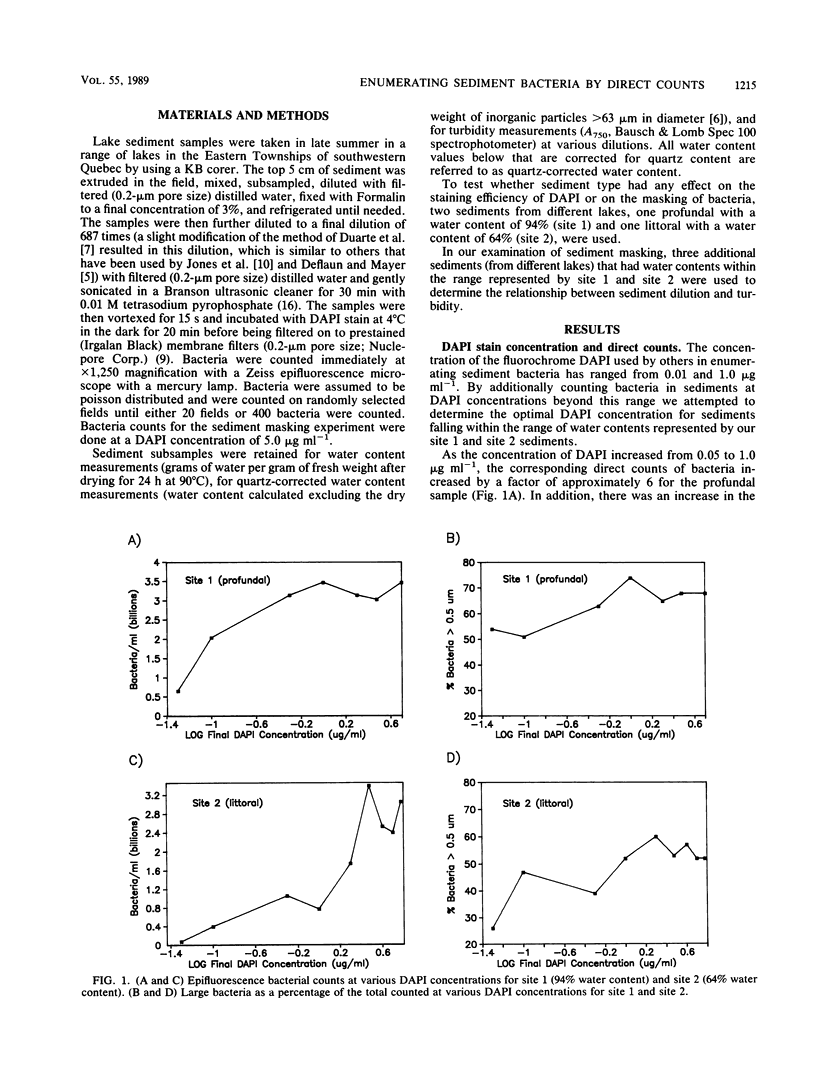
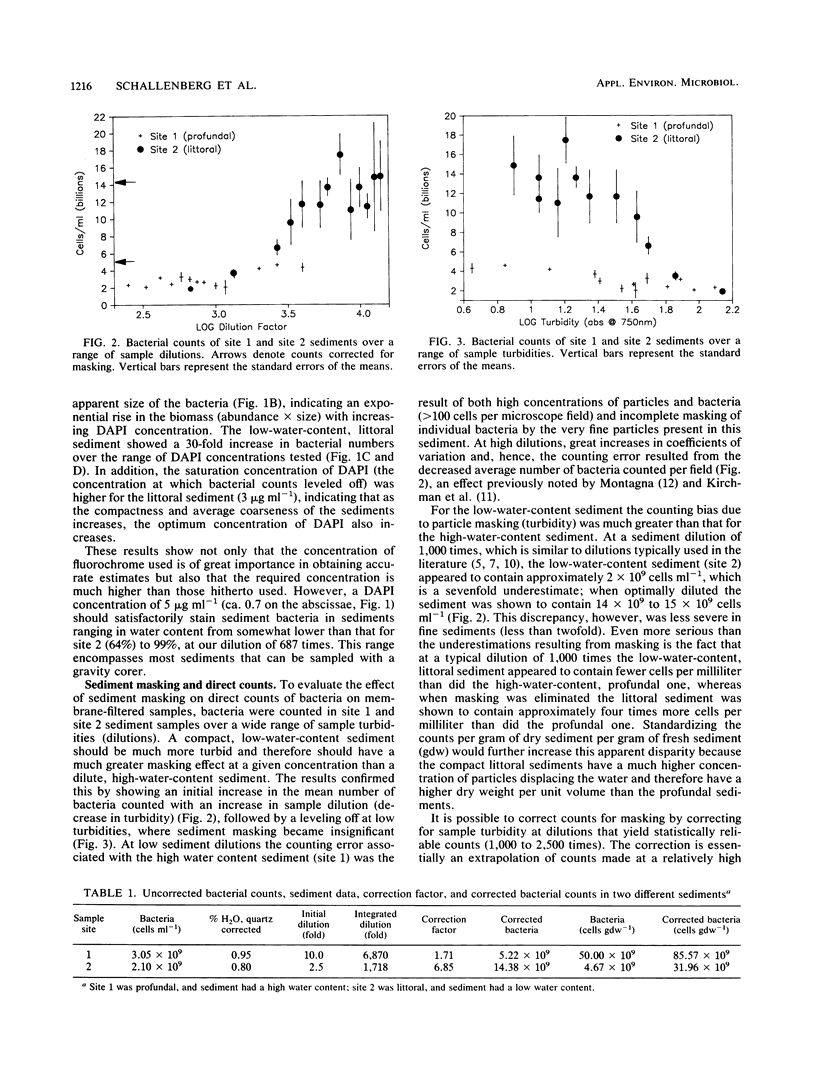
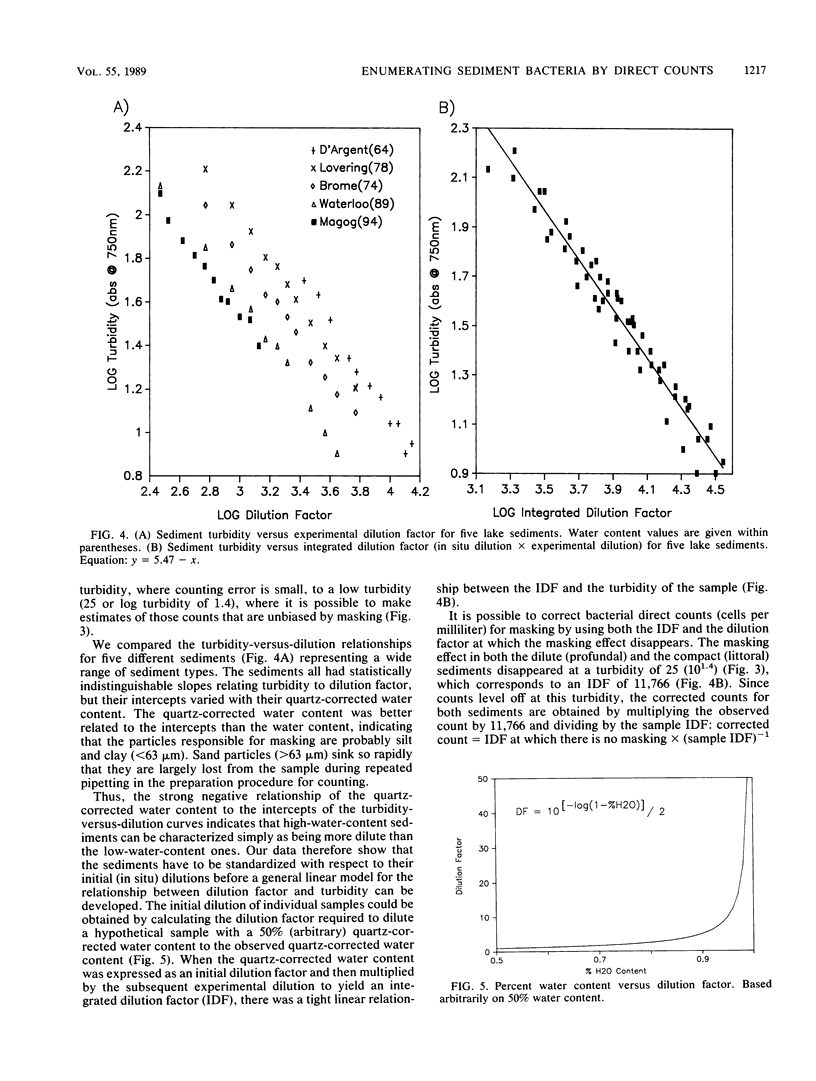
We examined the effect of different sediment types on the staining effectiveness of the fluorochrome DAPI (4′-6-diamidino-2-phenylindole dihydrochloride) over a wide range of concentrations and on the masking effect of sediment particles on DAPI-stained bacteria. Sediment type greatly affects the staining efficiency of DAPI, and most published studies seem to have underestimated bacterial abundances by using suboptimal concentrations of the fluorochrome. A DAPI concentration of 5 μg ml−1 is required to effectively stain the bacteria in most sediments that can be sampled with a gravity corer. When the sediments are diluted 687 times (a dilution factor similar to those most often used in the literature), sediment particle masking of stained bacteria is highly variable for different sediment types. By using a measure of turbidity (A750) to indicate masking and the quartz-corrected water content as a measure of the initial (in situ) dilution of each sediment type, it becomes possible to show a linear relationship between masking and the integrated (initial × experimental) dilution of various sediments. This relationship allows the development of a correction procedure for masking which makes accurate and unbiased counts possible. Data so obtained show a strong relationship between bacteria (cells per milliliter of fresh sediment) and sediment organic matter (grams [dry weight] per milliliter of fresh sediment), one that is not discernable without the correction. The proposed method of staining and correction for sediment masking provides the basis for a standardized interpretation of sediment bacterial counts.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bott T. L., Kaplan L. A. Bacterial biomass, metabolic state, and activity in stream sediments: relation to environmental variables and multiple assay comparisons. Appl Environ Microbiol. 1985 Aug;50(2):508–522. doi: 10.1128/aem.50.2.508-522.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke K. R., Joint I. R. Methodology for estimating numbers of free-living and attached bacteria in estuarine water. Appl Environ Microbiol. 1986 May;51(5):1110–1120. doi: 10.1128/aem.51.5.1110-1120.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchman D., Sigda J., Kapuscinski R., Mitchell R. Statistical analysis of the direct count method for enumerating bacteria. Appl Environ Microbiol. 1982 Aug;44(2):376–382. doi: 10.1128/aem.44.2.376-382.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagna P. A. Sampling design and enumeration statistics for bacteria extracted from marine sediments. Appl Environ Microbiol. 1982 Jun;43(6):1366–1372. doi: 10.1128/aem.43.6.1366-1372.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobell C. E. The Effect of Solid Surfaces upon Bacterial Activity. J Bacteriol. 1943 Jul;46(1):39–56. doi: 10.1128/jb.46.1.39-56.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]


