Abstract
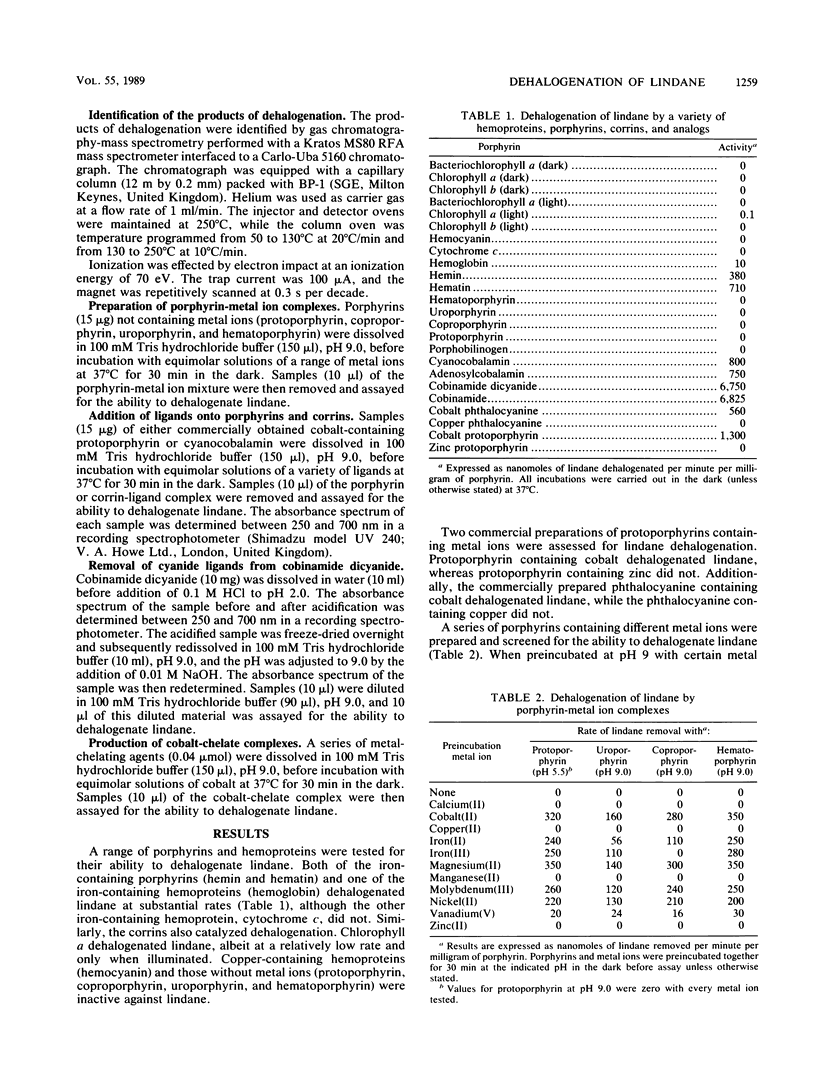
The dehalogenation of lindane by a range of hemoproteins, porphyrins, and corrins has been tested under reducing conditions in the presence of dithiothreitol. In addition, a series of porphyrin-metal ion complexes have been prepared and have also been screened for the capacity to dehalogenate lindane. Hemoglobin, hemin, hematin, and chlorophyll alpha all catalyzed the dehalogenation of lindane, as did all of the corrins tested. The porphyrins which did not contain metal centers--coproporphyrin, hematoporphyrin, protoporphyrin, and uroporphyrin--were inactive. However, when these porphyrins were then complexed with Co, Fe, Mg, Mo, Ni, or V, lindane dehalogenation was observed. In all cases, the reaction proceeded by an initial dechlorination to produce tetrachlorocyclohexene, which was further dehalogenated to yield chlorobenzene as the end product.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- FALK J. E., DRESEL E. I., BENSON A., KNIGHT B. C. Studies on the biosynthesis of blood pigments. 4. The nature of the porphyrins formed on incubation of chicken erythrocyte preparations with glycine, delta-aminolaevulic acid or porphobilinogen. Biochem J. 1956 May;63(1):87–94. doi: 10.1042/bj0630087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heritage A. D., Rae I. C. Identification of intermediates formed during the degradation of hexachlorocyclohexanes by Clostridium sphenoides. Appl Environ Microbiol. 1977 Jun;33(6):1295–1297. doi: 10.1128/aem.33.6.1295-1297.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagnow G., Haider K., Ellwardt P. C. Anaerobic dechlorination and degradation of hexachlorocyclohexane isomers by anaerobic and facultative anaerobic bacteria. Arch Microbiol. 1977 Dec 15;115(3):285–292. doi: 10.1007/BF00446454. [DOI] [PubMed] [Google Scholar]
- Khalifa S., Holmstead R. L., Casida J. E. Toxaphene degradation by iron(II) protoporphyrin systems. J Agric Food Chem. 1976 Mar-Apr;24(2):277–282. doi: 10.1021/jf60204a009. [DOI] [PubMed] [Google Scholar]
- Ohisa N., Yamaguchi M., Kurihara N. Lindane degradation by cell-free extracts of Clostridium rectum. Arch Microbiol. 1980 Apr;125(3):221–225. doi: 10.1007/BF00446880. [DOI] [PubMed] [Google Scholar]
- PORRA R. J., ROSS B. D. HAEM SYNTHASE AND COBALT PORPHYRIN SYNTHASE IN VARIOUS MICRO-ORGANISMS. Biochem J. 1965 Mar;94:557–562. doi: 10.1042/bj0940557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade R. S., Castro C. E. Oxidation of iron (II) porphyrins by alkyl halides. J Am Chem Soc. 1973 Jan 10;95(1):226–230. doi: 10.1021/ja00782a040. [DOI] [PubMed] [Google Scholar]
- Zoro J. A., Hunter J. M., Eglinton G., Ware G. C. Degradation of p,p'-DDT in reducing environments. Nature. 1974 Jan 25;247(5438):235–237. doi: 10.1038/247235a0. [DOI] [PubMed] [Google Scholar]



