Abstract
The pharmacological phenotype of ATP-sensitive potassium (KATP) channels is defined by their tissue-specific regulatory subunit, the sulfonylurea receptor (SUR), which associates with the pore-forming channel core, Kir6.2. The potassium channel opener diazoxide has hyperglycemic and hypotensive properties that stem from its ability to open KATP channels in pancreas and smooth muscle. Diazoxide is believed not to have any significant action on cardiac sarcolemmal KATP channels. Yet, diazoxide can be cardioprotective in ischemia and has been found to bind to the presumed cardiac sarcolemmal KATP channel-regulatory subunit, SUR2A. Here, in excised patches, diazoxide (300 μM) activated pancreatic SUR1/Kir6.2 currents and had little effect on native or recombinant cardiac SUR2A/Kir6.2 currents. However, in the presence of cytoplasmic ADP (100 μM), SUR2A/Kir6.2 channels became as sensitive to diazoxide as SUR1/Kir6.2 channels. This effect involved specific interactions between MgADP and SUR, as it required Mg2+, but not ATP, and was abolished by point mutations in the second nucleotide-binding domain of SUR, which impaired channel activation by MgADP. At the whole-cell level, in cardiomyocytes treated with oligomycin to block mitochondrial function, diazoxide could also activate KATP currents only after cytosolic ADP had been raised by a creatine kinase inhibitor. Thus, ADP serves as a cofactor to define the responsiveness of cardiac KATP channels toward diazoxide. The present demonstration of a pharmacological plasticity of KATP channels identifies a mechanism for the control of channel activity in cardiac cells depending on the cellular ADP levels, which are elevated under ischemia.
The potassium channel-opening drug (KCO) diazoxide has been in clinical use for the treatment of persistent hyperinsulinemia and hypertensive emergencies (1). Its pharmacodynamic properties have been related to the opening of ATP-sensitive potassium (KATP) channels present in pancreatic β-cells and smooth muscle, where these channels regulate insulin secretion and vascular tone (2, 3). Plasma membrane KATP channels are constituted of an inward rectifier K+ channel, Kir6.2, associated with a regulatory subunit, the sulfonylurea receptor (SUR) (4–6). SUR, a member of the ATP-binding-cassette (ABC) transporter family, is the primary target of the KCOs (7–9). It is now established that the nucleotide-binding domains of SUR are tightly linked to the sites of action of KCOs because binding and effects of KCOs require hydrolyzable nucleotides and are compromised by mutations that, in other ABC transporters, impair the ability of these domains to bind and hydrolyze nucleotides (8–11). Several isoforms of SUR have been identified, including SUR1 in pancreatic β-cells (4, 5) and SUR2B in smooth muscle (12).
In addition to its hyperglycemic and hypotensive properties, diazoxide also has the ability to protect ischemic myocardial tissue (13), which expresses SUR2A, the presumed cardiac isoform of SUR (14). However, it has been difficult to demonstrate any stimulatory effect of diazoxide on plasmalemmal cardiac KATP channels, either in their native form (15) or after reconstitution by heterologous coexpression of Kir6.2 and SUR2A (14, 16, 17). It has, therefore, become an established dogma that diazoxide does not act on cardiac sarcolemmal KATP channels (18–20). This has given weight to the hypothesis that diazoxide could produce some of its cardioprotective effects through activation of mitochondrial, rather than plasmalemmal, KATP channels (13, 21).
Binding experiments, however, have demonstrated that diazoxide can interact not only with SUR1, but also with SUR2A itself (8). Moreover, some reports have described a diazoxide-induced shortening of the cardiac action potential and/or an increase in whole-cell currents (13, 22, 23), suggesting that conditions exist where diazoxide could activate cardiac Kir6.2/SUR2A channels, much like pancreatic Kir6.2/SUR1 channels.
Here, we report that increase in cytoplasmic MgADP is the condition required for diazoxide to activate cardiac KATP channels, both native and recombinant. This implies that the transduction steps linking opener binding and channel opening are regulated by nucleotide interaction with the nucleotide-binding domains not only in the SUR1 protein (10, 11), but also in SUR2A. As the ischemic myocardium is characterized by increased levels of cytosolic ADP, an ADP-dependent activation of cardiac KATP channels by diazoxide could be relevant to its overall cardioprotective action.
A preliminary account of this work has been published in abstract form.¶
Materials and Methods
Xenopus Oocytes.
Mouse Kir6.2 (ref. 5; GenBank accession no. D50581), hamster SUR1 (ref. 4; GenBank accession no. L40623), and rat SUR2A (ref. 14; GenBank accession no. D83598) were subcloned in vectors derived from the Xenopus oocyte expression vector pGEMHE (24). After amplification and linearization, plasmid DNAs were transcribed in vitro by using the T7 mMessage mMachine kit (Ambion, Austin, TX). cRNAs were electrophoresed on formaldehyde gels, and concentrations were estimated from two dilutions by using RNA marker as a standard. Mutagenesis of SUR2A was done directly on the plasmid pGEMHE-SUR2A by PCR amplification of both DNA strands with complementary primers mutated to produce the desired amino acid change (QuickChange site-directed mutagenesis kit; Stratagene). The primers, 5′-CCGGTAGTGGGATGAGCTCTCTATCTCTGG-3′ for the K1348M mutation and 5′-GCAGCATACTGATCATGAATGAGGCCACGGCCTCC-3′ for D1469N, also incorporated the new restriction sites SacI and BclI to facilitate screening of the correct constructs. These constructs were sequenced to confirm the mutations.
Xenopus laevis were anaesthetized with 3-aminobenzoic acid ethyl ester (1 g/liter of water). Part of one ovary was removed, the incision was sutured, and the animal was allowed to recover. Stage V or VI oocytes were defolliculated by an ≈60-min incubation at 19°C with 2 mg/ml type A collagenase (Sigma). Selected oocytes were injected the next day with 50 nl of water containing ≈2 ng of Kir6.2 cRNA and with ≈6 ng of cRNA encoding SUR1 or SUR2A. They were stored at 19°C in a modified Barth’s solution with (in mM): 1 KCl, 0.82 MgSO4, 88 NaCl, 2.4 NaHCO3, 0.41 CaCl2, 0.3 Ca(NO3)2, and 16 Hepes (pH 7.4) supplemented with 100 units/ml penicillin, 100 μg/ml streptomycin, and 100 μg/ml gentamycin.
Three to 15 days after injection, oocytes were devitellinized, and heterologously expressed KATP channels were characterized by the patch-clamp technique in the excised inside-out configuration (25) by using techniques similar to those we used to record native frog skeletal muscle KATP channels (26, 27).
Conditions were designed to optimize recording of KATP currents and minimize contributions by endogenous oocyte Cl− currents. Patch pipettes (2–10 MΩ) contained (in mM) 154 K+, 146 Cl−, 5 Mg2+, and 10 piperazine-N,N′-bis(2-ethane-sulfonic acid) (Pipes)-KOH (pH 7.1). The cytoplasmic face of the patch was bathed in solutions that contained (in mM) 174 K+, 40 Cl−, 1 EGTA, 10 Pipes-KOH (pH 7.1), and methanesulfonate− as the remaining anions. Except where noted, Mg2+ was 1 mM. The membrane potential was maintained at −50 mV.
Cardiac Myocytes.
Guinea pig ventricular myocytes were isolated by enzymatic dissociation as described (28). For inside-out patch–clamp recordings, fire-polished pipettes (5–7 MΩ) contained (in mM): 140 KCl, 1 CaCl2, 1 MgCl2, 5 Hepes-KOH (pH 7.3), whereas the bath solution had (in mM): 140 KCl, 1 MgCl2, 5 EGTA, 5 Hepes-KOH (pH 7.3). Currents were monitored on-line on a high-gain digital storage oscilloscope (VC-6025; Hitachi, Tokyo) and stored on tape with a digital data recorder (VR-10; Instrutech, Mineola, NY). Currents were filtered off-line at 1 kHz with a Bessel filter (Frequency Devices 902; Haverhill, MA), sampled at 150-μs intervals, and analyzed by using the BIOQUEST software (28). For whole-cell recordings, cardiomyocytes were superfused with a Tyrode solution containing (in mM): 136.5 NaCl, 5.4 KCl, 1.8 CaCl2, 0.53 MgCl2, 5.5 glucose, and 5.5 Hepes-NaOH (pH 7.4). Pipettes (≈5 MΩ) for whole-cell recording contained (in mM): 140 KCl, 1 MgCl2, 2 ATP, 5 EGTA, and 10 Hepes-KOH (pH 7.3).
Materials.
ATP and ADP (potassium salts; Sigma), diazoxide (100 mM stock in DMSO; Sigma), oligomycin (a mixture of oligomycins A, B, and C; 1 mg/ml stock in DMSO; Sigma), and 2, 4-dinitro-1-fluorobenzene (DNFB; Fluka) were added as specified. All recordings were done at room temperature (≈22°C). Results are displayed as mean ± SEM.
Results
Activation of SUR2A/Kir6.2 by Diazoxide in the Presence of ADP.
Within 3 days after injection of Xenopus oocytes with cRNAs coding for Kir6.2 and either SUR1 or SUR2A, macroscopic KATP currents could be recorded in excised inside-out patches. SUR1 and SUR2A/Kir6.2 channels exhibited similar sensitivities to inhibition by intracellular ATP, with 10–20 μM ATP causing half-inhibition on average (9).
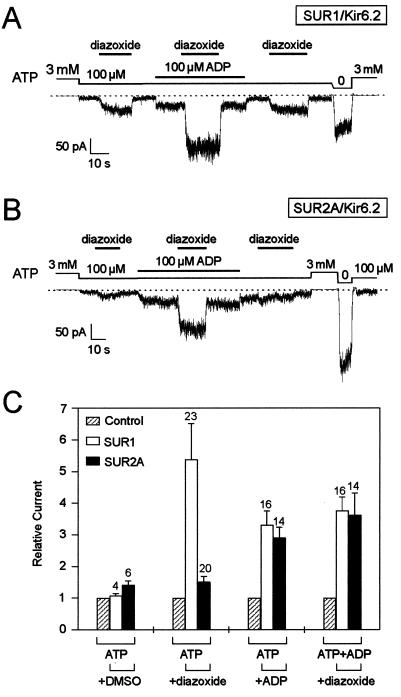
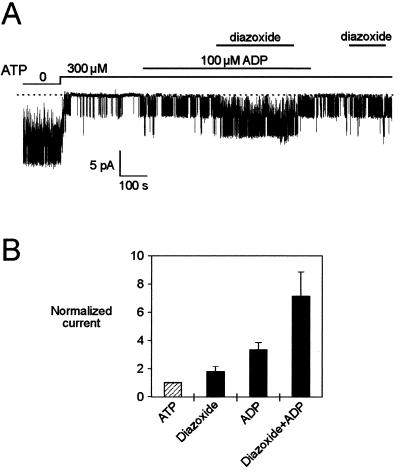
When currents were blocked to about 15% of their maximal values by 100 μM ATP, diazoxide (300 μM) caused a 5-fold increase in SUR1/Kir6.2 currents but had little effects on SUR2A/Kir6.2 currents (Fig. 1). However, in the presence of 100 μM ADP, diazoxide caused a robust increase in current, regardless of whether SUR1 or SUR2A was expressed. At 100 μM, ADP produced a 3-fold stimulation of ATP-inhibited SUR1 or SUR2A/Kir6.2 currents. On top of this stimulation, diazoxide further activated currents more than 3-fold (3.8 ± 0.4 for SUR1 and 3.6 ± 0.7 for SUR2A). Therefore, ADP uncovers a previously unrecognized ability of SUR2A/Kir6.2 channels to respond to diazoxide.
Figure 1.
Diazoxide activates SUR2A/Kir6.2 KATP channels in the presence of ADP. (A) Representative current trace recorded at −50 mV from an inside-out patch excised from an oocyte expressing Kir6.2 and SUR1. (B) Idem with SUR2A. (C) Average effects on SUR1/Kir6.2 and SUR2A/Kir6.2 currents of, from left to right, the vehicle DMSO, diazoxide in the absence of ADP, ADP, and diazoxide in the presence of ADP. For the latter, control represents the current measured in the presence of ADP. Numbers above bars indicate the number of patches tested. Responses were recorded as in A and B in the presence of 100 μM ATP and 1 mM Mg2+ by using 100 μM ADP, 300 μM diazoxide, and 0.3% DMSO.
Nucleotide Requirements for Diazoxide Activation of SUR2A/Kir6.2 Channels.
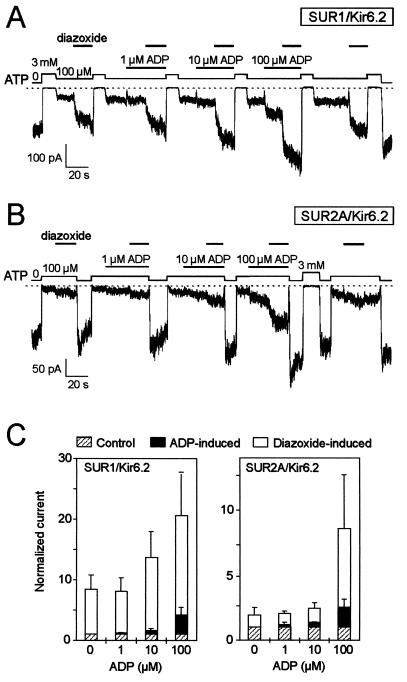
For SUR2A/Kir6.2 channels, there is a sharp dependence of the effect of diazoxide on the concentration of ADP (Fig. 2B), with channel activation by diazoxide becoming significant only at or above 100 μM ADP. For SUR1/Kir6.2 channels (Fig. 2A), diazoxide-induced activity is already vigorous in the absence of added ADP and is enhanced progressively by further addition of 10 and 100 μM ADP. For both channel isoforms, the sensitivity to ADP alone appeared equivalent, with slight activation at 10 μM and large activation at 100 μM (Fig. 2C).
Figure 2.
ADP concentration dependence of diazoxide activation of SUR2A/Kir6.2 KATP channels. (A) Effects of diazoxide (300 μM) at increasing concentrations of ADP on SUR1/Kir6.2 channels. (B) Idem with SUR2A. (C) Cumulative histograms showing the normalized currents induced by increasing concentrations of ADP and by diazoxide at each of those concentrations of ADP. Average data for SUR1/Kir6.2 (Left) and SUR2A/Kir6.2 (Right) were obtained from three patches each by using the protocols of A and B.
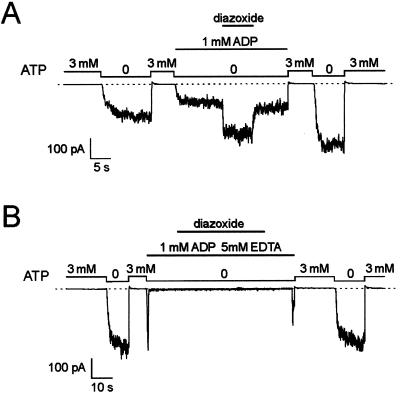
The experiments described in Figs. 1 and 2 were performed with both ATP and Mg2+ present. In the absence of ATP and presence of Mg2+ (1 mM), when the concentration of ADP was raised to 1 mM, which partially blocked KATP current, diazoxide (300 μM) activated SUR2A/Kir6.2 currents to the maximum level observed in the absence of nucleotides (Fig. 3A; fold increase = 2.03 ± 0.4; n = 5). After removal of Mg2+ and in the presence of EDTA, diazoxide could not increase channel activity despite the presence of 1 mM ADP (Fig. 3B), suggesting that MgADP, rather than ADP alone, is required for opener-induced activation of SUR2A/Kir6.2 channels.
Figure 3.
Diazoxide activation of SUR2A/Kir6.2 KATP channels does not require the presence of ATP but requires Mg2+. (A) Activation by diazoxide (300 μM) of SUR2A/Kir6.2 KATP channels in the presence of ADP (1 mM) as the only blocking nucleotide with 1 mM Mg2+. In five different patches, current in diazoxide relative to control was 2.03 ± 0.37. (B) Idem in the absence of Mg2+ with 5 mM EDTA to chelate contaminant Mg2+. Both traces are from the same patch.
Mutations in the Second NBD of SUR2A Eliminate ADP and Diazoxide Activation.
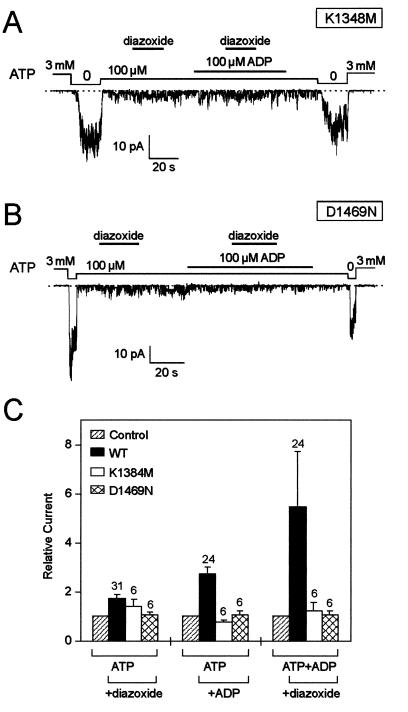
ATP-binding-cassette transporters contain two cytoplasmic NBDs, NBD1 and NBD2, with Walker A and B motifs forming an ATP-binding pocket (29). The highly conserved Walker A lysine and Walker B aspartate are important for ATP binding and/or hydrolysis, as the lysine residue interacts with the β-phosphate of ATP, whereas the aspartate residue coordinates with a Mg2+ ion to interact with the β- and γ-phosphates of ATP (30). In SUR1, mutations of these residues in both NBDs disrupt the channel response to ADP and diazoxide (10, 11). Mutations in NBD1 eliminate ATP binding, whereas equivalent mutations in NBD2 only impair the ability of MgADP to antagonize ATP binding (31), suggesting that the NBD2 domain is responsible for the interaction between SUR1 and MgADP. The role of NBD2 in MgADP-dependent diazoxide action on SUR2A/Kir6.2 channels was directly tested in channels with mutated Walker A lysine (K1348M) and Walker B aspartate (D1469N) residues. These two SUR2A mutants, when coexpressed with Kir6.2, produced functional channels with a sensitivity to ATP similar to that observed in the wild type (Fig. 4). However, these mutants produced channels with an impaired sensitivity to MgADP (Fig. 4), consistent with a critical role for NBD2 in mediating the interaction of ADP with SUR2A. In these mutants, the lack of sensitivity to ADP was associated with the inability of diazoxide to activate the channels alone or in the presence of MgADP (Fig. 4).
Figure 4.
Point mutations in Walker A and Walker B motifs of the second nucleotide binding domain of SUR2A impair the ability of ADP to promote diazoxide activation. (A) Lack of effect of diazoxide (300 μM) and ADP on KATP channels from an oocyte expressing wild-type Kir6.2 and mutated SUR2A with a K1348M mutation in the second Walker A motif. (B) Idem with the Walker B mutant D1469N of SUR2A. (C) Average effects of diazoxide, ADP, and diazoxide plus ADP on wild-type and mutant SUR2A/Kir6.2 currents.
Diazoxide Activates Native Cardiac KATP Channels.
SUR2A is the proposed cardiac isoform of SUR (14–17), and the ADP requirement for diazoxide-induced channel activation should be reproducible with the channel expressed in its native environment. Diazoxide was therefore tested on KATP channels in membranes of ventricular myocytes. Like SUR2A/Kir6.2 channels, native cardiac KATP channels were blocked by 300 μM ATP and activated by 100 μM ADP (Fig. 5A). In the presence of ADP, diazoxide (300 μM) produced significant increase in channel activity, which was only marginal in the absence of ADP. On average, the effect of diazoxide in the absence of ADP was equivalent in native channels and recombinant SUR2A/Kir6.2 channels (fold increases of 1.79 ± 0.35, n = 8, and 1.51 ± 0.14, n = 20, respectively). Such effect could be because of vehicle (see Fig. 1C) and/or possible presence of contaminant ADP in the ATP solutions. In the presence of ADP, diazoxide in native channels produced significant activation, albeit the relative increase in native channel activity was smaller than that observed in recombinant channels (fold increases of 2.15 ± 0.52, n = 8, vs. 3.56 ± 0.71, n = 14). This apparent difference in efficacy might not reflect a real variance in the responsiveness of native vs. recombinant channels. It could be because of saturation of the effect of diazoxide in native channels operating, in the presence of ADP, at levels beyond which even more effective activators could not produce a more pronounced channel activation.
Figure 5.
ADP promotes diazoxide activation of native cardiac KATP channels. (A) Current tracing recorded at −60 mV in an inside-out patch excised from a guinea pig ventricular myocyte. (B) Normalized current observed in eight patches in the presence of ADP (100 μM), diazoxide (300 μM), or both. To reduce errors because of low channel activity, mean current was estimated from nPo values (n, number of channels; Po, open probability) obtained by analysis of gating transitions (28).
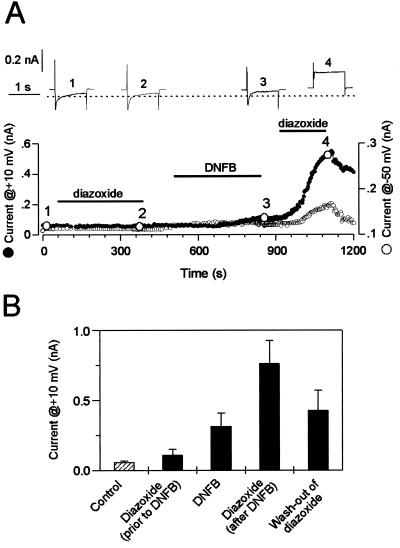
In contrast to inside-out patches, in the intact cardiomyocytes, KATP channels are coupled to intracellular ATP-consuming/ATP-generating systems (32). Under such conditions, the efficacy of diazoxide will depend on the availability of ADP at the channel site. In cardiomyocytes treated with the mitochondrial ATP-synthase inhibitor oligomycin, diazoxide did not activate KATP channel current (Fig. 6). This suggests that, in addition to mitochondrial ADP consumption, other catalytic activities recycle ADP into ATP. This may include creatine kinase, which is responsible for 90% of ATP turnover in cardiac cells (33). Therefore, we added to oligomycin-treated cardiomyocytes the creatine kinase inhibitor DNFB, which, on its own, induced modest channel activation (Fig. 6), suggesting that, with inhibited creatine kinase, levels of ADP were raised. Further application of diazoxide produced vigorous increase in outward current, in line with a diazoxide-induced activation of KATP current (Fig. 6). The diazoxide-induced outward current partially reversed on washout (Fig. 6 A and B) and was observed at both +10 mV, where outward and inward current components are not separated, and −50 mV, where the inward current component is essentially inactivated (Fig. 6A). Thus, after inhibition of cellular ADP-utilizing systems, diazoxide can enhance whole-cell KATP current.
Figure 6.
Diazoxide enhances whole-cell K+ current in cardiomyocytes after treatment with DNFB, a creatine kinase inhibitor. (A) Time course of the diazoxide (300 μM) response of steady state whole-cell outward currents before and after application of DNFB (100 μM). Under conditions of physiological ionic concentration gradients with internal 2 mM ATP, steady state currents were measured at the end of 1-s pulses to +10 mV from a holding potential of −50 mV (●). In addition, the baseline current measured at −50 mV is shown (○). Original current traces corresponding to numbered circles are shown as Insets. Cardiomyocytes were pretreated with oligomycin (1 mg/ml) for 5 min to disrupt mitochondrial function and prevent effects of diazoxide through mitochondria. (B) Average currents measured at +10 mV from eight cardiomyocytes.
Discussion
Diazoxide has a special place among KCOs, as it displays an apparently high selectivity for pancreatic β-cell and smooth muscle over cardiac KATP channels. In isolated patches from cardiac muscle cells or from cells expressing recombinant SUR2A/Kir6.2 channels, a stimulatory effect of diazoxide has never been reported to date, leading to the consensus opinion that cardiac sarcolemmal KATP channels are insensitive to this drug (18–21).
Present results demonstrate that diazoxide can produce activation of cardiac KATP channels with ADP serving as an essential cofactor in this phenomenon. Experimental evidence was obtained for both recombinant and native cardiac KATP channels. In the absence of ADP, diazoxide caused only a slight activation of cardiac KATP channels, which could have been overlooked in previous studies. This activation could have arisen from an effect of the vehicle, in our case DMSO, or from the presence of contaminant ADP, which cannot be avoided when solutions containing ATP are used. In the presence of 100 μM ADP, diazoxide produced a large activation comparable to that seen with pancreatic Kir6.2/SUR1 channels. The concentration threshold for this effect of ADP was between 10 and 100 μM. This could explain the lack of clear effects of diazoxide on membrane currents from resting myocytes where the concentration of free ADP is maintained below the micromolar range by a number of ADP-utilizing processes, including the creatine kinase reaction (34). Only after blocking ADP consumption could we observe a consistent increase in whole-cell KATP currents by diazoxide. To ensure that diazoxide acted directly on sarcolemmal channels and not indirectly via mitochondrial channels (13, 21, 35), in those experiments, cells were pretreated with oligomycin to disrupt mitochondrial function. The evidence thus shows that diazoxide will increase K+ currents in heart muscle only in conditions of elevated ADP levels as might occur under ischemic conditions (35). This preference for sarcolemmal channels in metabolically compromised cells, combined with the activation of mitochondrial channels (13, 21, 36), could contribute to the cardioprotective efficacy of diazoxide.
In terms of molecular structure, the observed correlation between native channels and SUR2A/Kir6.2 supports the hypothesis that these two entities are identical (14, 16, 37). Experiments showing that diazoxide can displace the binding of a radiolabeled opener from SUR2A (8) imply that SUR2A possesses a diazoxide binding site like other known isoforms of the SUR do. As binding experiments (8) were done in the absence of ADP, it would appear that ADP facilitates diazoxide activation by acting on the coupling between diazoxide and channel opening, rather than on the binding step. That Mg2+ is required, but ATP is neither sufficient nor necessary to promote diazoxide action suggests, first, that ADP, produced through a possible (though still undemonstrated) hydrolysis of ATP by SUR, cannot replace exogenously supplied ADP, and second, that the mechanism involves the second NBD, NBD2, of SUR2A, which preferentially binds MgADP (31). Corroborating this latter conclusion, mutations of the conserved lysine (to methionine) and aspartate (to asparagine) residues of the Walker A and B motifs of NBD2 abolished both ADP and diazoxide activation. In SUR1, the corresponding lysine-to-methionine mutation cancelled the activatory effects of ADP but not of diazoxide (10), suggesting that an obligatory link between ADP and diazoxide exists in SUR2A but not SUR1.
In summary, we provide evidence for the critical role of intracellular ADP in defining the response of cardiac KATP channel toward diazoxide. That cardiac KATP channels can be rendered diazoxide-sensitive provides a mechanistic basis for the action of this opener on cardiac sarcolemmal KATP channels. Along with previous reports of nucleotide diphosphate-dependent action of channel openers (10, 11, 38), as well as blockers (29, 39–42), the present study demonstrates a fundamental role for ADP in defining the pharmacological plasticity of KATP channels.
Acknowledgments
We are grateful to Dr. J. Bryan (Houston, TX) for hamster SUR1, Dr. S. Seino (Chiba, Japan) for mouse Kir6.2 and rat SUR2A, and Dr. D. Logothetis (New York, NY) for providing vector pGEMHE. This work was made possible by grants from Association Francaise contre les Myopathies, Association Francaise de Lutte contre la Mucoviscidose with additional support provided by Commissariat à l’Energie Atomique, Centre National de la Recherche Scientifique, the Miami Heart Research Institute, and the Ruth and Bruce Rappaport Program in Vascular Biology and Gene Delivery at the Mayo Foundation. N.D., H.J., and C.M. were supported by a fellowship from La Société des Amis des Sciences, a studentship from Association pour la Recherche contre le Cancer, and a studentship from La Ligue contre le Cancer, respectively. A.E.A. is a recipient of a fellowship from the American Heart Association, Northland Affiliate.
Abbreviations
- KCO
potassium channel opener
- KATP channel
ATP-sensitive potassium channel
- SUR
sulfonylurea receptor
- DNFB
2,4-dinitro-1-fluorobenzene
- NBD
nucleotide-binding domain
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
Moreau, C., D’hahan, N., Jacquet, H., Alekseev, A. E., Terzic, A. & Vivaudou, M. (1999) Biophys. J. 76, A413.
References
- 1.Paulissian R. Int Anesthesiol Clin. 1978;16:201–237. doi: 10.1097/00004311-197816020-00010. [DOI] [PubMed] [Google Scholar]
- 2.Trube G, Rorsman P, Ohno-Shosaku T. Pflügers Arch Eur J Physiol. 1986;407:493–499. doi: 10.1007/BF00657506. [DOI] [PubMed] [Google Scholar]
- 3.Standen N B, Quayle J M, Davies N W, Brayden J E, Huang Y, Nelson M T. Science. 1989;245:177–180. doi: 10.1126/science.2501869. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Bryan L, Nichols C G, Wechsler S W, Clement J P, Boyd A E, Gonzalez G, Herrerasosa H, Nguy K, Bryan J, Nelson D A. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- 5.Inagaki N, Gonoi T, Clement J P, Namba N, Inazawa J, Gonzalez G, Aguilar-Bryan L, Seino S, Bryan J. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- 6.Lorenz E, Alekseev A E, Krapivinsky G B, Carrasco A J, Clapham D E, Terzic A. Mol Cell Biol. 1998;18:1652–1659. doi: 10.1128/mcb.18.3.1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tucker S J, Gribble F M, Zhao C, Trapp S, Ashcroft F M. Nature (London) 1997;387:179–183. doi: 10.1038/387179a0. [DOI] [PubMed] [Google Scholar]
- 8.Schwanstecher M, Sieverding C, Dorschner H, Gross I, Aguilar-Bryan L, Schwanstecher C, Bryan J. EMBO J. 1998;17:5529–5535. doi: 10.1093/emboj/17.19.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D’hahan N, Jacquet H, Moreau C, Catty P, Vivaudou M. Mol Pharmacol. 1999;56:308–315. doi: 10.1124/mol.56.2.308. [DOI] [PubMed] [Google Scholar]
- 10.Gribble F M, Tucker S J, Ashcroft F M. EMBO J. 1997;16:1145–1152. doi: 10.1093/emboj/16.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shyng S L, Ferrigni T, Nichols C G. J Gen Physiol. 1997;110:643–654. doi: 10.1085/jgp.110.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Isomoto S, Kondo C, Yamada M, Matsumoto S, Higashiguchi O, Horio Y, Matsuzawa Y, Kurachi Y. J Biol Chem. 1996;271:24321–24324. doi: 10.1074/jbc.271.40.24321. [DOI] [PubMed] [Google Scholar]
- 13.Garlid K D, Paucek P, Yarov-Yarovoy V, Murray H N, Darbenzio R B, D’Alonzo A J, Lodge N J, Smith M A, Grover G J. Circ Res. 1997;81:1072–1082. doi: 10.1161/01.res.81.6.1072. [DOI] [PubMed] [Google Scholar]
- 14.Inagaki N, Gonoi T, Clement J P, Wang C Z, Aguilar-Bryan L, Bryan J, Seino S. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- 15.Faivre J F, Findlay I. Biochim Biophys Acta. 1989;984:1–5. doi: 10.1016/0005-2736(89)90334-9. [DOI] [PubMed] [Google Scholar]
- 16.Babenko A P, Gonzalez G, Aguilar-Bryan L, Bryan J. Circ Res. 1998;83:1132–1143. doi: 10.1161/01.res.83.11.1132. [DOI] [PubMed] [Google Scholar]
- 17.Okuyama Y, Yamada M, Kondo C, Satoh E, Isomoto S, Shindo T, Horio Y, Kitakaze M, Hori M, Kurachi Y. Pflügers Arch Eur J Physiol. 1998;435:595–603. doi: 10.1007/s004240050559. [DOI] [PubMed] [Google Scholar]
- 18.Isomoto S, Kurachi Y. J Cardiovasc Electrophysiol. 1997;8:1431–1446. doi: 10.1111/j.1540-8167.1997.tb01040.x. [DOI] [PubMed] [Google Scholar]
- 19.Aguilar-Bryan L, Clement J P, Gonzalez G, Kunjilwar K, Babenko A, Bryan J. Physiol Rev. 1998;78:227–245. doi: 10.1152/physrev.1998.78.1.227. [DOI] [PubMed] [Google Scholar]
- 20.Seino S. Annu Rev Physiol. 1999;61:337–362. doi: 10.1146/annurev.physiol.61.1.337. [DOI] [PubMed] [Google Scholar]
- 21.Liu Y, Sato T, O’Rourke B, Marban E. Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 22.Ciampolillo F, Tung D E, Cameron J S. J Pharmacol Exp Ther. 1992;260:254–260. [PubMed] [Google Scholar]
- 23.Sato T, Wu B, Nakamura S, Kiyosue T, Arita M. Br J Pharmacol. 1993;108:549–556. doi: 10.1111/j.1476-5381.1993.tb12839.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liman E R, Tytgat J, Hess P. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- 25.Hamill O P, Marty A, Neher E, Sakmann B, Sigworth F J. Pflügers Arch Eur J Physiol. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 26.Vivaudou M, Forestier C. J Physiol (London) 1995;486:629–645. doi: 10.1113/jphysiol.1995.sp020840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forestier C, Pierrard J, Vivaudou M. J Gen Physiol. 1996;107:489–502. doi: 10.1085/jgp.107.4.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Alekseev A E, Brady P A, Terzic A. J Gen Physiol. 1998;111:381–394. doi: 10.1085/jgp.111.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Higgins C F. Annu Rev Cell Biol. 1992;8:67–113. doi: 10.1146/annurev.cb.08.110192.000435. [DOI] [PubMed] [Google Scholar]
- 30.Hung L W, Wang I X, Nikaido K, Liu P Q, Ames G F-L, Kim S H. Nature (London) 1998;396:703–707. doi: 10.1038/25393. [DOI] [PubMed] [Google Scholar]
- 31.Ueda K, Inagaki N, Seino S. J Biol Chem. 1997;272:22983–22986. doi: 10.1074/jbc.272.37.22983. [DOI] [PubMed] [Google Scholar]
- 32.Dzeja P P, Terzic A. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- 33.Dzeja P P, Vitkevicius K T, Redfield M M, Burnett J C, Terzic A. Circ Res. 1999;84:1137–1143. doi: 10.1161/01.res.84.10.1137. [DOI] [PubMed] [Google Scholar]
- 34.Radda G K. Science. 1986;233:640–645. doi: 10.1126/science.3726553. [DOI] [PubMed] [Google Scholar]
- 35.Holmuhamedov E L, Jovanovic S, Dzeja P P, Jovanovic A, Terzic A. Am J Physiol. 1998;44:H1567–H1576. doi: 10.1152/ajpheart.1998.275.5.H1567. [DOI] [PubMed] [Google Scholar]
- 36.Holmuhamedov E L, Wang L, Terzic A. J Physiol (London) 1999;519:347–360. doi: 10.1111/j.1469-7793.1999.0347m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lorenz E, Terzic A. J Mol Cell Cardiol. 1999;31:425–434. doi: 10.1006/jmcc.1998.0876. [DOI] [PubMed] [Google Scholar]
- 38.Jahangir A, Terzic A, Kurachi Y. Cardiovasc Res. 1994;28:831–835. doi: 10.1093/cvr/28.6.831. [DOI] [PubMed] [Google Scholar]
- 39.Venkatesh N, Lamp S T, Weiss J N. Circ Res. 1991;69:623–637. doi: 10.1161/01.res.69.3.623. [DOI] [PubMed] [Google Scholar]
- 40.Terzic A, Findlay I, Hosoya Y, Kurachi Y. Neuron. 1994;12:1049–1058. doi: 10.1016/0896-6273(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 41.Jovanovic A, Zhang S C, Alekseev A E, Terzic A. Pflügers Arch Eur J Physiol. 1996;431:800–802. doi: 10.1007/BF02253848. [DOI] [PubMed] [Google Scholar]
- 42.Brady P A, Alekseev A E, Terzic A. Circ Res. 1998;82:272–278. doi: 10.1161/01.res.82.2.272. [DOI] [PubMed] [Google Scholar]