Abstract
Dent’s disease is an X-linked inherited disorder characterized by hypercalciuria, nephrocalcinosis, nephrolithiasis, low molecular weight proteinuria, Fanconi’s syndrome, and renal failure. It is caused by inactivating mutations in CLC5, a member of the CLC voltage-gated chloride channel family. CLC5 is known to be expressed in the endosomal compartment of the renal proximal tubule, where it may be required for endosomal acidification and trafficking. Although the Fanconi’s syndrome and low molecular weight proteinuria in Dent’s disease can be explained by disruption of endosomal function in this nephron segment, the pathogenesis of the hypercalciuria in this disease is unknown. We have generated transgenic mice (RZ) with reduced CLC5 expression by introduction of an antisense ribozyme targeted against CLC5. RZ mice are markedly hypercalciuric compared with nontransgenic control mice, at a time when their serum electrolytes and renal function are otherwise normal. This suggests that hypercalciuria in Dent’s disease is a direct consequence of CLC5 hypofunction and is not attributable to a gain of function by mutant CLC5, an effect of modifier genes, or a secondary result of nonspecific renal injury. Surprisingly, hypercalciuria in RZ mice is abolished by dietary calcium deprivation, suggesting that the hypercalciuria may be attributable to gastrointestinal hyperabsorption of calcium rather than a renal calcium leak.
Idiopathic hypercalciuria is the most common metabolic risk factor for the development of kidney stones and is eight times more prevalent in the stone-forming population than among normal individuals. It therefore poses a significant clinical problem. The primary abnormality that causes hypercalciuria is unknown. Both a renal calcium leak and intestinal calcium hyperabsorption may play a role. Because of the complex nature of hypercalciuria, encompassing environmental and polygenic factors, the study of individual mechanisms contributing to calciuria has been difficult. Recently, Dent’s disease, a monogenic disorder manifesting with hypercalciuria, nephrolithiasis, Fanconi’s syndrome, low molecular weight proteinuria, renal insufficiency, and rickets, has been mapped to the gene encoding CLC5, a renal chloride channel of unknown function (1–5). Expression of mutant CLC5 in Xenopus oocytes results in reduction or abolition of chloride currents compared with wild-type CLC5, suggesting that loss of CLC5 function may play a direct role in the pathogenesis of Dent’s disease (3). Although Dent’s disease itself is rare, it provides a simple model for hypercalciuria. Elucidating the mechanism of hypercalciuria in this disorder may provide useful insight into the pathogenesis of idiopathic hypercalciuria in the broader population.
Two observations suggest that the primary defect in Dent’s disease originates in the kidney. First, in humans, CLC5 is expressed almost exclusively in the kidney (6–8). Second, nephrolithiasis and nephrocalcinosis does not recur in Dent’s patients who receive a renal transplant from a normal individual, suggesting that their hypercalciuria may be abolished (9, 10). However, although serum calcium remains normal in Dent’s disease, 1,25-dihydroxyvitamin D levels are frequently elevated, and parathyroid hormone levels are often low, findings that are not consistent with primary renal calcium wasting (10, 11). Furthermore, some patients clearly exhibit intestinal hyperabsorption of a dietary calcium load (11). The insights into the pathophysiology of Dent’s disease that can be provided by the study of patients are limited because of heterogeneity of clinical presentation. Many patients already have renal insufficiency at the time of diagnosis. Conversely, some “patients” are clinically asymptomatic children that have been identified by mass screening (4, 12).
We and others have recently demonstrated that CLC5 is primarily expressed in endosomes of the proximal convoluted tubule and, to a lesser extent, in medullary thick ascending limb (13–15). These findings lend weight to the hypothesis that defective CLC5 function might cause disruption of endosomal function by impairing adequate endosomal acidification (16). Disruption of endocytosis and membrane transport protein recycling in proximal tubular epithelial cells could conceivably explain the renal tubular reabsorptive dysfunction manifesting as low molecular weight proteinuria and Fanconi’s syndrome in Dent’s disease. However, CLC5 was not expressed in cortical thick ascending limb, distal convoluted tubule, or connecting segment, the primary sites for transepithelial calcium reabsorption and its regulation (17). Thus, the proximate cause of the hypercalciuria in these patients remains unclear.
To investigate the origins of the hypercalciuria in Dent’s disease, we have generated a mouse model with reduced CLC5 expression by introduction of a CLC5-inactivating antisense ribozyme transgene. We find that these mice are hypercalciuric compared with nontransgenic control mice, at a time when their serum electrolytes and renal function are otherwise normal. This suggests that hypercalciuria in Dent’s disease is a direct consequence of CLC5 hypofunction and not attributable to a gain of function by mutant CLC5, the effect of modifier genes, or a secondary result of nonspecific renal injury. The hypercalciuria in RZ mice is abolished by dietary calcium deprivation, suggesting that the hypercalciuria may be attributable to gastrointestinal hyperabsorption of calcium rather than a renal calcium leak. These results raise the possibility of a novel role for CLC5 in the control of intestinal calcium absorption.
Methods
Design of Ribozymes and in Vitro Catalytic Assay.
By homology-based reverse transcription–PCR of mouse kidney, a 604-bp cDNA containing part of the coding sequence of the mouse CLC5 ortholog (MCL5A, 96% identity to nucleotides 838–1,440 of rat CLC5, GenBank accession no. Z56277) was isolated. Similarly, 764- and 921-bp cDNAs encompassing overlapping homologous regions of mouse CLC4 (MCL4A, 100% identity to nucleotides 1,035–1,798 of mouse CLC4, GenBank accession no. Z49916) and CLC3 (MCL3A, 96% identity to nucleotides 1,171–2,092 of rat CLC3, GenBank accession no. D17521), respectively, also were isolated. The region within the mouse CLC5 sequence sharing the least similarity to CLC3 and CLC4, and containing a central UUC nucleotide triplet, was selected as the target for an antisense hammerhead ribozyme, RZ (Fig. 1A). The RZ ribozyme sequence (Fig. 1B) consisted of two 10-nt flanking complementary regions that were designed to be antisense to the CLC5 mRNA, linked together by a 22-nt conserved catalytic sequence identical to that used in other ribozymes (18–20). Two controls were prepared. First, a ribozyme was designed in which the sequence of the flanking regions was inverted to the sense sequence but the catalytic domain remained intact (SZ). Second, a molecule was designed in which the catalytic domain was inverted but the flanking complementary regions were preserved. This molecule would be expected to hybridize to CLC5 mRNA but have no catalytic activity (NC). cDNAs encoding RZ, SZ, and NC each were constructed from overlapping oligonucleotides and were cloned into the plasmid vector pBluescript II KS(+) (Stratagene).
Figure 1.
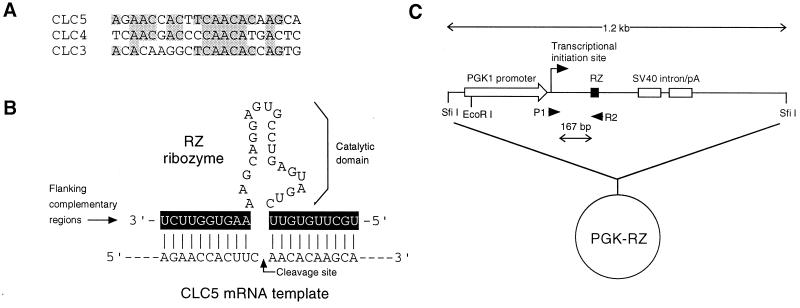
Design of the antisense ribozyme RZ. (A) Alignment of the sequences of mouse CLC5 with CLC3 and CLC4 in the region targeted for ribozyme cleavage, showing limited sequence similarity. (B) Sequence of the hammerhead ribozyme RZ. The flanking complementary regions (shaded) are antisense to and would be expected to hybridize with CLC5 mRNA, thus allowing the catalytic domain to attack and cleave CLC5 at the site indicated by the arrow, just downstream of a UUC triplet. (C) Map of the PGK-RZ plasmid construct used to express RZ in transgenic mice.
To test the efficacy and specificity of RZ, an in vitro RNA cleavage assay was performed. Radiolabeled MCL5A, MCL4A, and MCL3A cRNA templates were prepared by in vitro transcription from their plasmid clones using T3 or T7 RNA polymerase in the presence of α-thio[35S]ATP. Unlabeled ribozyme and control cRNAs were synthesized similarly by using unlabeled ribonucleotides. For each assay, 10 nM template cRNA and a specified amount of ribozyme cRNA were added together to a buffer containing 20 mM MgCl2 and 50 mM Tris⋅HCl (pH 8) denatured at 65°C for 5 min, cooled slowly, and incubated at 37°C, typically for 1 hr. The reaction was terminated by placing on ice and adding an equal volume of stop solution (95% formamide/20 mM EDTA/0.05% xylene cyanol/0.05% bromophenol blue), then was denatured at 85°C for 5 min and was electrophoresed on a 4% polyacrylamide/8 M urea denaturing gel, and the labeled bands were visualized by autoradiography. To determine the activity of each band accurately, the corresponding region of the gel was cut out for liquid scintillation counting.
Generation of Ribozyme-Transgenic Mice.
The PGK-RZ construct was generated by insertion of the RZ cDNA into the eukaryotic expression plasmid vector pEMBLpgkSV [a generous gift of Ivan Rodriguez, Centre Médical Universitaire, Geneva (21)], so that it was flanked by an upstream mouse phosphoglycerate kinase (PGK1) promoter, which is known to direct constitutive and ubiquitous expression in transgenic mice (22), and a downstream intron polyadenylation signal of the SV40 T gene (Fig. 1C). Linearized PGK-RZ was excised by digestion with SfiI and was injected into FVB mouse embryos, and transgenic mice were generated according to established procedures (23).
Genotyping of Transgenic Mice.
Genomic DNA was isolated from tail biopsies of weanlings by standard methods (23) and was genotyped by PCR using primers P1 and R2 (Fig. 1C). The presence of the transgene was confirmed on PCR-positive tail DNA by Southern blot analysis. Thirty micrograms of tail DNA was digested overnight with EcoRI in the presence of RNase A, was electrophoresed on a 0.75% agarose gel for 15 hr, and was blotted overnight to a nylon membrane by capillary transfer. A digoxigenin-labeled antisense riboprobe was synthesized by in vitro transcription of the ribozyme transgene construct using T7 RNA polymerase and digoxigenin-labeled UTP. Membranes were hybridized at high stringency with the riboprobe and chemiluminescent detection performed by using the Genius System (Boehringer Mannheim).
Breeding and Maintenance of Mice.
Founder transgenic mice were bred with co-isogenic normal mice of the FVB strain (Taconic Farms, NY) to obtain heterozygous F1 offspring on a pure FVB background. All subsequent studies were performed with these heterozygous F1 mice, designated RZ mice. Normal, nontransgenic FVB mice were used as controls. For baseline studies, mice were allowed free access to tap water and standard Laboratory Rodent Diet (Ralston Purina), which contains 0.95% calcium (Ca), 0.4% phosphorus (P), and 4.5 units/g vitamin D. For dietary manipulation studies, mice were allowed free access to tap water and a normal calcium diet (TD 97191, Harlan Teklad, Madison, WI, containing 0.6% Ca, 0.4% P, 2.2 units/g vitamin D) or deionized water and a low calcium diet (TD 95027, Harlan Teklad, containing <0.02% Ca, 0.4% P, 2.2 units/g vitamin D). During the overnight fast, mice had no access to chow but had free access to deionized water. All cages were kept under identical light conditions with a 12-hr light-dark cycle. At the time of death, mice were anesthetized by intraperitoneal injection of sodium pentobarbital. Whole blood was obtained by cardiac puncture, and the serum was separated and analyzed for a standard panel of electrolytes (Tufts Veterinary Diagnostic Laboratory, North Grafton, MA). Kidneys were removed, were snap frozen in liquid nitrogen, and were stored at −80°C until used for RNA or protein extraction.
Reverse Transcription–PCR and Northern Blot Analysis.
Total RNA was isolated from RZ and control kidneys by the guanidinium isothiocyanate-CsCl method (24). Expression of the RZ transgene was analyzed by reverse transcription–PCR, using the primers P1 and R2. Duplicate cDNA samples were amplified by PCR with primers to β-actin, a house-keeping gene, as a positive control. In negative controls, in which reverse transcriptase was omitted, all PCR reactions were negative (data not shown). Northern blots were performed as described (14), using mouse CLC5, CLC4, and CLC3 riboprobes synthesized by in vitro transcription from MCL5A, MCL4A, and MCL3A.
Western Blot Analysis.
Expression of CLC5 protein was determined by Western blotting as described (14) with the exception that a 100,000 × g membrane preparation was used. In brief, RZ and control kidneys were homogenized and were centrifuged at 1,000 × g to pellet the nuclei, and the supernatant was further centrifuged at 100,000 × g to yield a microsomal membrane pellet. Fifty micrograms of this membrane protein fraction then was electrophoresed on a 7.5% SDS-polyacrylamide gel and was immunoblotted with a 1:100 dilution of C2 (14), a polyclonal antiserum raised against rat CLC5 that also cross-reacts with mouse CLC5. To assess the expression level of the homologous isoform, CLC3, an affinity-purified polyclonal anti-CLC3 antibody was purchased from Alomone Labs (Jerusalem) and was used at a 1:300 dilution. This antibody has previously been shown to be highly isoform-specific (25). We confirmed its specificity in our own hands by immunoblotting with control mouse brain microsomal membranes, which revealed a broad band of 100–115 kDa, known to represent the glycosylated form of the protein, that was blocked when the antibody was preincubated with CLC3 glutathione S-transferase fusion protein (data not shown). Uniform gel loading of membrane-associated proteins was confirmed by subsequently immunoblotting the membranes with an anti-actin antibody (Sigma) at 1: 200 dilution.
Measurement of Urine Chemistries.
Spot urines were collected from age- and sex-matched individual RZ and control mice and were assayed on the day of collection at room temperature. During dietary calcium manipulation, spot urines were obtained from each mouse between 10 and 11 am on the day of collection. Urine calcium concentration was measured by the calcium-cresolphthalein colorimetric assay according to the manufacturer’s instructions (Sigma), except that the assay volume was scaled down to 1 ml, requiring 10 μl of undiluted urine. Urine creatinine concentration was measured by using the alkaline-picrate colorimetric assay according to the manufacturer’s instructions (Sigma), scaled down to an assay volume of 1 ml, requiring 100 μl of urine diluted 1:15 in deionized water. The ratio of urine calcium to creatinine concentration was used as an index of calciuria, as has previously been validated (26–28).
Statistical Analysis.
Data were expressed as means ± SEM. Statistical significance was assessed by using the two-tailed t test, with Bonferroni’s correction where appropriate.
Results and Discussion
Use of a Transgenic Ribozyme Approach To Generate Knockdown Mice.
To generate a mouse model with an inherited defect in CLC5 function that might mimic Dent’s disease in humans, we made mice transgenic for a hammerhead ribozyme targeted against murine CLC5 mRNA. Ribozymes are RNA molecules that can act as enzymes. Hammerhead ribozymes are ribozymes that catalyze cleavage of other RNA molecules. The specificity of the ribozyme is conferred by the flanking complementary regions that are antisense to, and hybridize with, their cognate target (Fig. 1B). Thus, in mice transgenic for a CLC5-specific ribozyme, CLC5 mRNA would be cleaved, and CLC5 protein expression would be expected to be reduced or knocked down. This approach to ablation of gene expression, which has been used successfully in previous transgenic experiments (18–20), has several potential advantages over conventional gene knockout by homologous recombination in embryonic stem cells, including a reduced risk of the embryonic lethality that often results if a developmentally essential gene is completely knocked out, the potential for spatial and temporal regulation of ribozyme effect by using different constitutive or inducible promoters, and relative speed and ease of generation of the mice.
Design and in Vitro Testing of a CLC5-Specific Ribozyme.
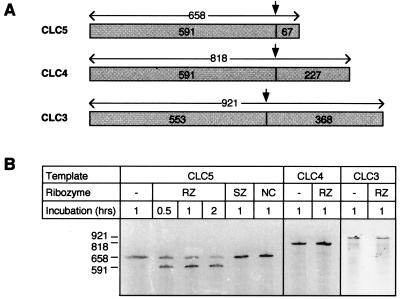
An antisense hammerhead ribozyme, RZ, was designed that would recognize and cleave within a 21-bp sequence containing a UUC triplet in the coding region of the mouse CLC5 mRNA (Fig. 1B). The homologous regions of CLC3 and CLC4, the closest homologs in the CLC gene family, share identity at only 11 residues and lack the central (G/U)UC triplet that is required for efficient cleavage (Fig. 1A). Thus, RZ would not be expected to hybridize to, or cleave, CLC3 and CLC4 mRNA and is likely to be highly specific for CLC5. In an in vitro assay, RZ was highly effective at cleaving CLC5 RNA, resulting in a >80% reduction in intact RNA after 2 hr (Fig. 2). This effect was highly specific because no cleavage occurred with either a sense ribozyme (SZ), in which the flanking complementary regions were inverted, or a noncatalytic ribozyme (NC) in which the catalytic domain was inverted. Furthermore, RZ could be shown to be isoform-specific because it did not cleave CLC3 or CLC4 RNA.
Figure 2.
In vitro ribozyme assay. (A) Diagram of CLC5, CLC4, and CLC3 cRNA templates. The predicted ribozyme cleavage site (arrow) and the expected sizes (in nucleotides) of the intact and cleaved fragments for CLC5, and the homologous regions of CLC3 and CLC4, are indicated. (B) Autoradiograph of the products of in vitro ribozyme cleavage after gel separation. Cleavage is observed only with the combination of CLC5 template and RZ ribozyme, which yield the expected 591-nt band, and an additional 67-nt fragment not visible on this gel.
Generation of Ribozyme-Transgenic Mice.
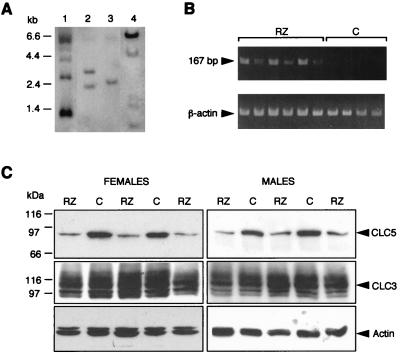
A cDNA encoding RZ was cloned into the eukaryotic expression vector, pEMBLpgkSV, under control of the constitutively active PGK promoter (Fig. 1C) and was used to generate transgenic mice. From a total of 16 pups, four potential founder mice were identified by analysis of tail DNA for the presence of the transgene. The founder mice were bred with normal FVB mice, and heterozygous F1 offspring were identified by both PCR and Southern blot (Fig. 3A). All four founders showed germline transmission. The Southern blot pattern was different for each founder, indicating that the transgene integration site in the four lines was different. Expression of RZ RNA transcripts was confirmed by reverse transcription–PCR of kidney total RNA (Fig. 3B).
Figure 3.
Characterization of RZ transgenic mice. (A) Southern blot of tail DNA from offspring of four different founder mice after EcoRI digestion, indicating different sites of transgene insertion in each line. (B) Reverse transcription–PCR of cDNA from RZ and control (C) mouse kidneys with P1 and R2 primers, demonstrating the presence of 167-bp RZ RNA transcripts only in the transgenic mice. The β-actin control is positive in all kidneys. (C) Western blots of mouse kidney membranes with antibodies against CLC5, CLC3, and actin, showing marked reduction of CLC5 protein levels in male and female RZ mice compared with controls and no difference in CLC3 or membrane-associated actin protein levels.
Knockdown of CLC5 Expression in RZ Mice.
We expected that expression of RZ in the kidneys of the transgenic mice would cause a reduction in intact CLC5 mRNA and hence a reduction in CLC5 protein expression. To assess the relative abundance of CLC5 RNA, high stringency Northern blotting was performed on total kidney RNA isolated from RZ and control mice, using the mouse MCL5A riboprobe. The CLC5 probe hybridized to a single band of 9.5 kilobases, but the intensity of this band was not reduced in RZ compared with control mouse kidneys (data not shown). Similarly, there was no reduction in CLC3 or CLC4 mRNA in RZ versus control mouse kidneys.
To determine whether CLC5 protein expression was reduced by the presence of the ribozyme, microsomal membranes were isolated from RZ and control mouse kidneys and were immunoblotted with the C2 antibody, which we have previously shown to be specific for CLC5 (14). Renal CLC5 protein expression was markedly reduced in the majority of RZ mice examined (n = 43), compared with controls (n = 29). Representative Western blots are shown in Fig. 3C. These results indicate that the presence of the ribozyme does lead to reduction in CLC5 protein levels, even though no reduction in CLC5 mRNA was detected, presumably by interfering with protein translation. Duplicate kidney membranes were immunoblotted with an anti-CLC3 antibody to confirm CLC5-specificity of the ribozyme effect. These demonstrated that there was no reduction in CLC3 protein levels in RZ compared with control mice (Fig. 3C). The ribozyme-associated reduction in CLC5 protein levels therefore appears to be isoform-specific.
Hypercalciuria in RZ Mice.
RZ mice were normal in appearance and survive normally up to 36 weeks of age. Body weights of both male and female RZ mice were similar to control mice at all ages (Fig. 4A). There was also no difference in a standard panel of serum chemistries, including calcium and inorganic phosphate (Table 1).
Figure 4.
Phenotype of RZ transgenic mice. (A) Body weights in RZ and control mice, stratified by age and sex. (B) Urine Ca/Cr in mice of different ages (∗, P < 0.01). (C) Individual urine Ca/Cr in mice of all ages, stratified by sex. Horizontal bars indicate means ± standard errors (∗, P < 0.02).
Table 1.
Serum chemistries in RZ and control mice
| RZ; n = 14 | Control; n = 8 | |
|---|---|---|
| Urea nitrogen, mg/dl | 29.5 ± 1.7 | 26.4 ± 1.6 |
| Creatinine, mg/dl | 0.11 ± 0.01 | 0.11 ± 0.02 |
| Phosphate, mg/dl | 7.9 ± 0.2 | 8.2 ± 0.5 |
| Calcium, mg/dl | 8.71 ± 0.09 | 8.63 ± 0.13 |
| Total protein, g/dl | 4.98 ± 0.15 | 4.62 ± 0.17 |
| Albumin, g/dl | 3.25 ± 0.10 | 2.88 ± 0.19 |
| Sodium, meq/liter | 152.4 ± 0.6 | 151.6 ± 0.9 |
| Potassium, meq/liter | 5.0 ± 0.2 | 5.1 ± 0.1 |
| Chloride, meq/liter | 111.9 ± 1.0 | 115 ± 1.1 |
| Total CO2, meq/liter | 17.8 ± 0.8 | 17.8 ± 1.9 |
In humans, hypercalciuria is a prominent finding in male hemizygotes with Dent’s disease and also is found in some female heterozygote carriers. To evaluate whether our knockdown mice were hypercalciuric, urine calcium-to-creatinine ratios (Ca/Cr) were determined from spot urine samples (Fig. 4C). Urine Ca/Cr was significantly higher (P < 0.003) in RZ (0.080 ± 0.003 mg/mg) compared with control mice (0.065 ± 0.003 mg/mg), when mice of all ages and gender were analyzed in aggregate. There was no difference in the degree of hypercalciuria between mice from the four different transgenic lines, indicating that hypercalciuria was not an artifact of gene disruption at any one integration site. Our results support the contention that the hypercalciuria in Dent’s disease is a direct result of a reduction in functional CLC5 and not attributable to a gain of function by a mutant CLC5, the effect of modifier genes, or a secondary consequence of nonspecific renal injury.
Normal female mice were found to have higher urine Ca/Cr than normal males, as has been previously noted in other mouse strains (27). When our data were stratified by gender, the calciuric effect of the ribozyme transgene was most prominent in males (P < 0.002, RZ vs. control, Fig. 4C). In females, presumably because the baseline urine Ca/Cr was already high, the trend for RZ mice to have higher urine Ca/Cr than controls did not reach statistical significance (P = 0.18, RZ vs. control, Fig. 4C). The gender dependence of expression of the hypercalciuric phenotype in RZ mice could not be explained by a gender difference in degree of knockdown of CLC5 protein expression (Fig. 3C). Interestingly, RZ mice exhibit an age-dependent decline in urine Ca/Cr such that by 18 weeks of age they were no different from controls (Fig. 4B). The reason for this age-dependent decline is still unclear.
Hypercalciuria in RZ Mice Depends on Dietary Calcium.
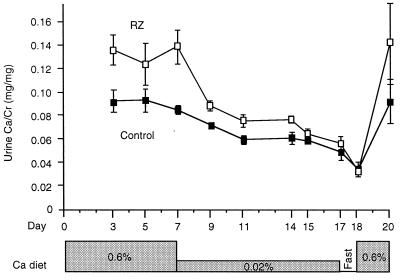
To evaluate whether hypercalciuria in RZ mice is of renal or absorptive origin, a dietary calcium deprivation test was performed on a cohort of 6- to 8-week-old male RZ (n = 14) and control (n = 13) mice (Fig. 5). On a normal calcium diet (0.6% Ca), urine Ca/Cr (average of three determinations from days 3–7) was 45% higher in RZ than control mice (P < 0.003). On a low calcium diet (<0.02% Ca), urine Ca/Cr fell in both RZ and control mice, as expected. However, the decline was much greater in RZ mice (urine Ca/Cr 14% higher than controls on day 17, P < 0.01). After an overnight fast, urine calcium excretion in RZ and control mice reached the same nadir and were indistinguishable (P = 0.67). On restoration of normal calcium diet, urine Ca/Cr ratios increased in both RZ and control mice, reestablishing their respective baselines. The body weights of mice in the two groups were no different throughout the study, indicating that there were no gross differences in feeding patterns.
Figure 5.
Dietary calcium deprivation test. Serial measurements of urine Ca/Cr were performed in mice placed on diets with different calcium content (indicated in the shaded boxes) or after an overnight fast.
It is not surprising that urine calcium excretion would be reduced in all mice on a low calcium diet because the filtered load of calcium would be expected to fall, and parathyroid hormone to rise. However, if RZ mice had a fixed defect in renal tubule calcium reabsorption, one would expect that, although calcium excretion might decrease with dietary calcium deprivation, it would always be higher than that of controls. Thus, the convergence of renal calcium excretion to the same low level in both groups of mice after dietary calcium deprivation argues for the absence of a fixed renal calcium leak in the RZ mice. This finding strongly suggests that the hypercalciuria in RZ mice is attributable instead to intestinal hyperabsorption of calcium. Alternatively, it remains possible that RZ mice might have a renal tubule calcium reabsorption disorder that is in some way attenuated by the low calcium diet.
Some but not all patients with CLC5 mutations have renal phosphate wasting, which may be associated with hypophosphatemia (11, 12, 29, 30). In two such patients, the hypercalciuria corrected with phosphate supplementation alone, suggesting that hypercalciuria was caused by hypophosphatemia (29). However, this is unlikely to be the mechanism of hypercalciuria in our RZ mice because their serum phosphate was not reduced. Thus, hypophosphatemia may be a phenotypic variant that occurs only in humans with specific gain-of-function mutations in CLC5, or specific modifier gene alleles, that are not present in our mice. Alternatively, it is possible that there may be more fundamental differences in the physiology of calcium and phosphate handling between mice and humans.
If indeed the hypercalciuria in RZ mice is attributable to intestinal hyperabsorption of calcium, two general mechanisms for this are possible. First, the hyperabsorption of calcium could be secondary to an elevated 1,25-dihydroxyvitamin D level, as has been demonstrated in some Dent’s patients (11, 30). If so, it raises the intriguing possibility that the role of CLC5 might be to regulate production of 1,25-dihydroxyvitamin D in the renal proximal tubule, either by regulation of the delivery of the substrate, 25-hydroxyvitamin D, which, as has recently been reported, (31) is endocytosed from the tubule lumen, or by directly regulating the 1 α-hydroxylase enzyme. Alternatively, because CLC5 is also expressed throughout the mouse intestinal epithelium (V.A.L., unpublished observations), it may directly regulate the expression of calcium transport proteins responsible for transepithelial absorption of calcium across the intestinal mucosa.
In conclusion, we have shown that introduction of a CLC5-specific ribozyme transgene in mice leads to reduction of renal CLC5 protein expression and consequent diet-dependent hypercalciuria. Although the exact mechanism remains obscure, our findings indicate that the hypercalciuria of Dent’s disease is a direct result of loss of CLC5 function. Furthermore, they raise the possibility of a role for CLC5 in the regulation of intestinal calcium absorption.
Acknowledgments
This work was supported by a Joseph E. Murray Research Award from the National Kidney Foundation of Massachusetts and Rhode Island and a Carl W. Gottschalk Research Scholar Award from the American Society of Nephrology (to A.Y.).
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Oudet C, Martin-Coignard D, Pannetier S, Praud E, Champion G, Hanauer A. Hum Genet. 1997;99:781–784. doi: 10.1007/s004390050448. [DOI] [PubMed] [Google Scholar]
- 2.Nakazato H, Hattori S, Furuse A, Kawano T, Karashima S, Tsuruta M, Yoshimuta J, Endo F, Matsuda I. Kidney Int. 1997;52:895–900. doi: 10.1038/ki.1997.410. [DOI] [PubMed] [Google Scholar]
- 3.Lloyd S E, Pearce S H S, Fisher S E, Steinmeyer K, Schwappach B, Scheinman S J, Harding B, Bolino A, Devoto M, Goodyer P, et al. Nature (London) 1996;379:445–449. doi: 10.1038/379445a0. [DOI] [PubMed] [Google Scholar]
- 4.Lloyd S E, Pearce S H S, Gunther W, Kawaguchi H, Igarashi T, Jentsch T J, Thakker R V. J Clin Invest. 1997;99:967–974. doi: 10.1172/JCI119262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fisher S E, van Bakel I, Lloyd S E, Pearce S H S, Thakker R V, Craig I W. Genomics. 1995;29:598–606. doi: 10.1006/geno.1995.9960. [DOI] [PubMed] [Google Scholar]
- 6.Fisher S E, Black G C M, Lloyd S E, Hatchwell E, Wrong O, Thakker R V, Craig I W. Hum Mol Genet. 1994;3:2053–2059. [PubMed] [Google Scholar]
- 7.Steinmeyer K, Schwappach B, Bens M, Vandewalle A, Jentsch T J. J Biol Chem. 1995;270:31172–31177. doi: 10.1074/jbc.270.52.31172. [DOI] [PubMed] [Google Scholar]
- 8.Sakamoto H, Kawasaki M, Uchida S, Sasaki S, Marumo F. J Biol Chem. 1996;271:10210–10216. doi: 10.1074/jbc.271.17.10210. [DOI] [PubMed] [Google Scholar]
- 9.Frymoyer P A, Scheinman S J, Dunham P B, Jones D B, Hueber P, Schroeder E T. N Engl J Med. 1991;325:681–686. doi: 10.1056/NEJM199109053251003. [DOI] [PubMed] [Google Scholar]
- 10.Scheinman S J. Kidney Int. 1998;53:3–17. doi: 10.1046/j.1523-1755.1998.00718.x. [DOI] [PubMed] [Google Scholar]
- 11.Reinhart S C, Norden A G W, Lapsley M, Thakker R V, Pang J, Moses A M, Frymoyer P A, Favus M J, Hoepner J A, Scheinman S J. J Am Soc Nephrol. 1994;5:1451–1461. doi: 10.1681/ASN.V571451. [DOI] [PubMed] [Google Scholar]
- 12.Wrong O M, Norden A G W, Feest T G. Q J Med. 1994;87:473–493. [PubMed] [Google Scholar]
- 13.Gunther W, Luchow A, Cluzeaud F, Vandewalle A, Jentsch T J. Proc Natl Acad Sci USA. 1998;95:8075–8080. doi: 10.1073/pnas.95.14.8075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Luyckx V A, Goda F O, Mount D M, Nishio T, Hall A, Hebert S C, Hammond T G, Yu A S L. Am J Physiol. 1998;275:F761–F769. doi: 10.1152/ajprenal.1998.275.5.F761. [DOI] [PubMed] [Google Scholar]
- 15.Devuyst O, Christie P T, Courtoy P J, Beauwens R, Thakker R V. Hum Mol Genet. 1999;8:247–257. doi: 10.1093/hmg/8.2.247. [DOI] [PubMed] [Google Scholar]
- 16.Hebert S C. Nature (London) 1996;379:398–399. doi: 10.1038/379398a0. [DOI] [PubMed] [Google Scholar]
- 17.Friedman P A, Gesek F A. Am J Physiol. 1993;264:F181–F198. doi: 10.1152/ajprenal.1993.264.2.F181. [DOI] [PubMed] [Google Scholar]
- 18.Zhao J J, Pick L. Nature (London) 1993;365:448–451. doi: 10.1038/365448a0. [DOI] [PubMed] [Google Scholar]
- 19.Efrat S, Leiser M, Wu Y-J, Fusco-Demane D, Emran O A, Surana M, Jetton T L, Magnuson M A, Weir G, Fleischer N. Proc Natl Acad Sci USA. 1994;91:2051–2055. doi: 10.1073/pnas.91.6.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Chen X, Wagner T E. Proc Natl Acad Sci USA. 1997;94:13777–13781. doi: 10.1073/pnas.94.25.13777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martinou J-C, Dubois-Dauphin M, Staple J K, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C, Huarte J. Neuron. 1994;13:1017–1030. doi: 10.1016/0896-6273(94)90266-6. [DOI] [PubMed] [Google Scholar]
- 22.Pravtcheva D D, Adra C N, Ruddle F H. Development (Cambridge, UK) 1991;111:1109–1120. doi: 10.1242/dev.111.4.1109. [DOI] [PubMed] [Google Scholar]
- 23.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the Mouse Embryo: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1994. [Google Scholar]
- 24.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Biochemistry. 1979;18:5294. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 25.Schmeider S, Lindenthal S, Idelson G H, Ehrenfeld J. J Physiol. 1999;517P:15P. (abstr.). [Google Scholar]
- 26.Shevrin D H, Lad T E, Kukreja S C. J Bone Miner Res. 1987;2:297–301. doi: 10.1002/jbmr.5650020406. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura H, Kawata H, Takahashi F, Higuchi Y, Furiuchi T, Ohkawa H. Am J Pathol. 1995;147:1682–1692. [PMC free article] [PubMed] [Google Scholar]
- 28.Alon U, Donaldson D L, Hellerstein S, Warady B A, Harris D J. J Pediatr. 1992;120:899–905. doi: 10.1016/s0022-3476(05)81957-2. [DOI] [PubMed] [Google Scholar]
- 29.Bolino A, Devoto M, Enia G, Zoccali C, Weissenbach J, Romero G. Eur J Hum Genet. 1993;1:269–279. doi: 10.1159/000472424. [DOI] [PubMed] [Google Scholar]
- 30.Bosio M, Bianchi M L, Lloyd S E, Thakker R V. Pediatr Nephrol. 1999;13:278–283. doi: 10.1007/s004670050608. [DOI] [PubMed] [Google Scholar]
- 31.Nykjaer A, Dragun D, Walther D, Vorum H, Jacobsen C, Herz J, Melsen F, Christensen E I, Willnow T E. Cell. 1999;96:507–515. doi: 10.1016/s0092-8674(00)80655-8. [DOI] [PubMed] [Google Scholar]