Abstract
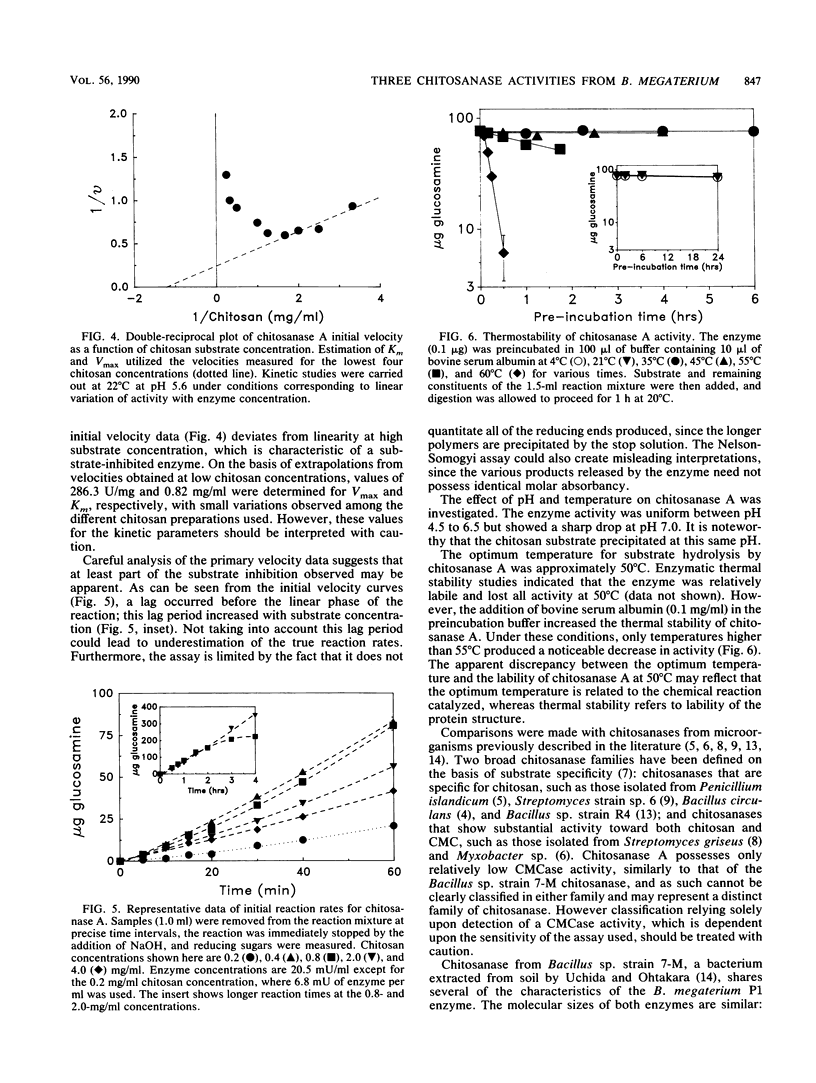
Bacillus megaterium P1, a bacterial strain capable of hydrolyzing chitosan, was isolated from soil samples. Chitosan-degrading activity was induced by chitosan but not by its constituent d-glucosamine. Extracellular secretion of chitosanase reached levels corresponding to 1 U/ml under optimal conditions. Three chitosan-degrading proteins (chitosanases A, B, and C) were purified to homogeneity. Chitosanase A (43 kilodaltons) was highly specific for chitosan and represented the major chitosan-hydrolyzing species. Chitosanases B (39.5 kilodaltons) and C (22 kilodaltons) corresponded to minor activities and possessed comparable specific activities toward chitosan, chitin, and cellulose. Chitosanase A was active from pH 4.5 to 6.5 and was stable on the basis of activity up to 45°C. The optimum temperature for enzymatic chitosan hydrolysis was 50°C. Kinetic studies on chitosanase A suggest that the enzyme is substrate inhibited. The apparent Km and Vmax determined at 22°C and pH 5.6 were 0.8 mg/ml and 280 U/mg, respectively. End products of chitosan hydrolysis by each of the three chitosanases were identified as glucosamine oligomers, similar to those obtained for previously reported chitosanase digestions.
Full text
PDF

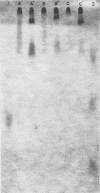
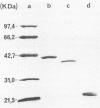
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hedges A., Wolfe R. S. Extracellular enzyme from Myxobacter AL-1 that exhibits both beta-1,4-glucanase and chitosanase activites. J Bacteriol. 1974 Nov;120(2):844–853. doi: 10.1128/jb.120.2.844-853.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KREGER D. R. Observations on cell walls of yeasts and some other fungi by x-ray diffraction and solubility tests. Biochim Biophys Acta. 1954 Jan;13(1):1–9. doi: 10.1016/0006-3002(54)90264-4. [DOI] [PubMed] [Google Scholar]
- Ohtakara A. Chitosanase from Streptomyces griseus. Methods Enzymol. 1988;161:505–510. doi: 10.1016/0076-6879(88)61067-6. [DOI] [PubMed] [Google Scholar]
- Price J. S., Storck R. Production, purification, and characterization of an extracellular chitosanase from Streptomyces. J Bacteriol. 1975 Dec;124(3):1574–1585. doi: 10.1128/jb.124.3.1574-1585.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saul A., Don M. A rapid method of concentrating proteins in small volumes with high recovery using Sephadex G-25. Anal Biochem. 1984 May 1;138(2):451–453. doi: 10.1016/0003-2697(84)90838-8. [DOI] [PubMed] [Google Scholar]
- Tominaga Y., Tsujisaka Y. Purification and some enzymatic properties of the chitosanase from Bacillus R-4 which lyses Rhizopus cell walls. Biochim Biophys Acta. 1975 Nov 20;410(1):145–155. doi: 10.1016/0005-2744(75)90215-6. [DOI] [PubMed] [Google Scholar]