Abstract
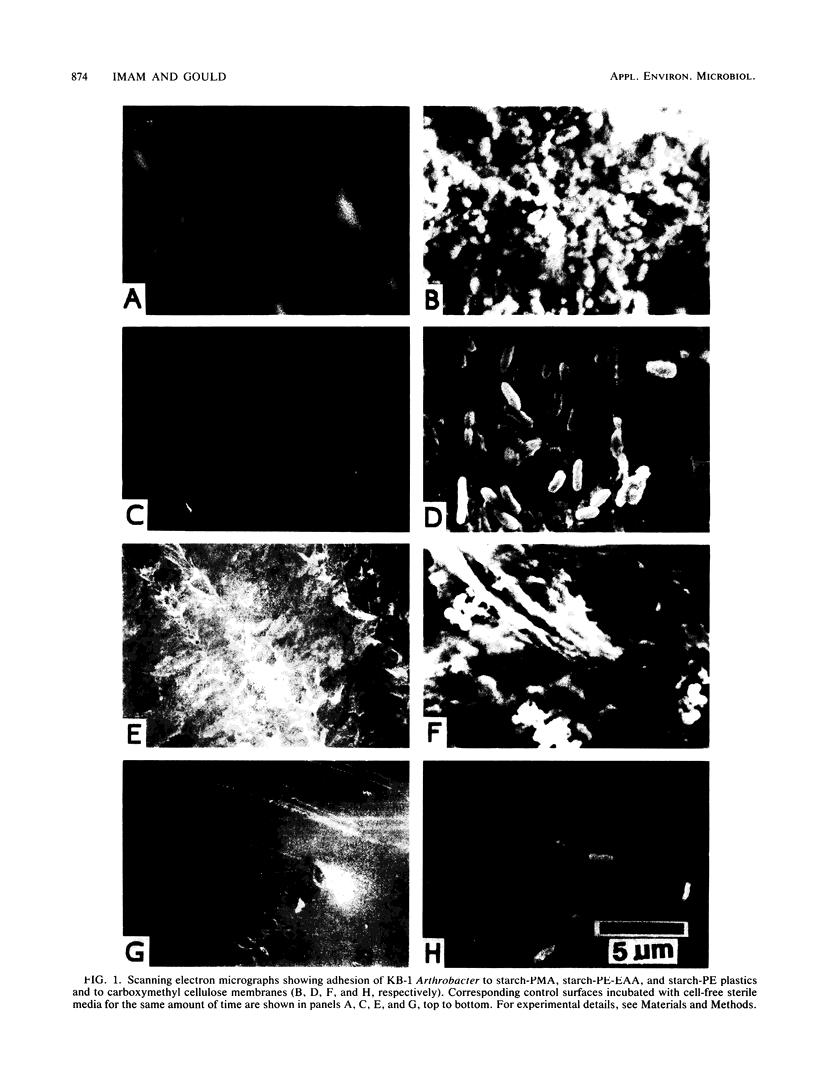
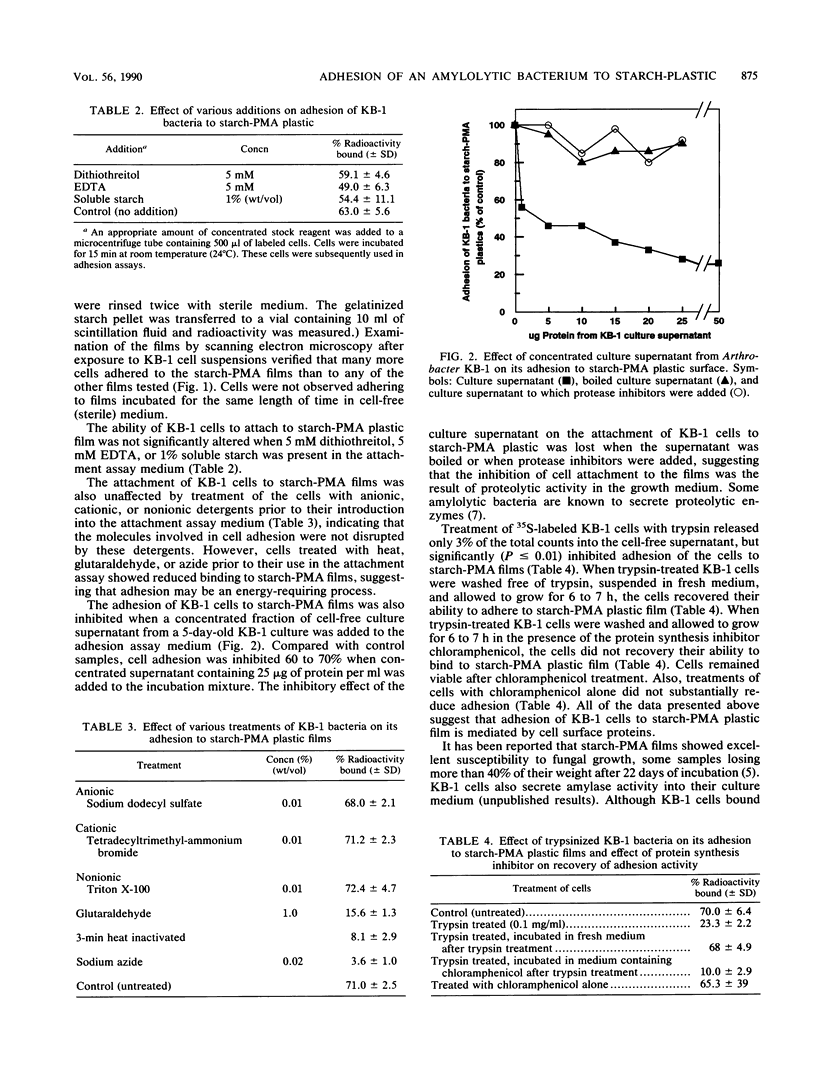
Cells of the amylolytic bacterium KB-1 (thought to be an Arthrobacter sp.) adhered (∼70%) to the surface of plastic films composed of starch-poly (methylacrylate) graft copolymer (starch-PMA), but did not adhere (<10%) to films composed of polymethylacrylate (PMA), polyethylene (PE), carboxymethyl cellulose, or a mixture of PE plus poly (ethylene-coacrylic acid) (EAA), starch plus PE, or starch plus PE and EAA. About 30% of the cells adhered to gelatinized insoluble starch. Dithiothreitol (5 mM), EDTA (5 mM), and soluble starch (1%, wt/vol) had little effect on the adhesion of KB-1 cells to starch-PMA films. However, glutaraldehyde-fixed cells, azide-treated cells, and heat-killed cells did not bind to starch-PMA plastic, suggesting that the observed adhesion required cell viability. Culture supernatant from 5-day-old KB-1 cultures contained a proteolytic enzyme that inhibited cell adhesion to starch-PMA plastics. Trypsin-treated KB-1 cells also lost their ability to bind to starch-PMA plastic. When washed free of trypsin and suspended in fresh medium, trypsin-treated bacteria were able to recover adhesion activity in the absence, but not in the presence, of the protein synthesis inhibitor chloramphenicol. These results suggested that adhesion of KB-1 to starch-PMA plastic may be mediated by a cell surface protein. Although KB-1 bacteria bound to starch-PMA plastic, they did not appear to degrade starch in these films. Evidence of starch degradation was observed for starch-PE-EAA plastics, where <10% of the bacteria was bound, suggesting that cell adhesion may not be a prerequisite for degradation of some starch-containing plastics.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson K. L., Salyers A. A. Biochemical evidence that starch breakdown by Bacteroides thetaiotaomicron involves outer membrane starch-binding sites and periplasmic starch-degrading enzymes. J Bacteriol. 1989 Jun;171(6):3192–3198. doi: 10.1128/jb.171.6.3192-3198.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit Z., Ofek I., Goldman R., Mirelman D., Sharon N. Mannose residues on phagocytes as receptors for the attachment of Escherichia coli and Salmonella typhi. Biochem Biophys Res Commun. 1977 Sep 9;78(1):455–460. doi: 10.1016/0006-291x(77)91276-1. [DOI] [PubMed] [Google Scholar]
- Friedberg E. C., Bonura T., Love J. D., McMillan S., Radany E. H., Schultz R. A. The repair of DNA damage: recent developments and new insights. J Supramol Struct Cell Biochem. 1981;16(1):91–103. doi: 10.1002/jsscb.1981.380160109. [DOI] [PubMed] [Google Scholar]
- Imam S. H., Bard R. F., Tosteson T. R. Specificity of marine microbial surface interactions. Appl Environ Microbiol. 1984 Oct;48(4):833–839. doi: 10.1128/aem.48.4.833-839.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imam S. H., Snell W. J. Degradation of the framework of the Chlamydomonas cell wall by proteases present in a commercially available alpha-amylase preparation. Appl Environ Microbiol. 1987 Jul;53(7):1701–1704. doi: 10.1128/aem.53.7.1701-1704.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr T. J., Kerr R. D., Benner R. Isolation of a bacterium capable of degrading peanut hull lignin. Appl Environ Microbiol. 1983 Nov;46(5):1201–1206. doi: 10.1128/aem.46.5.1201-1206.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson D. L., Rosen S. D., Barondes S. H. Discoidin, a developmentally regulated carbohydrate-binding protein from Dictyostelium discoideum. Purification and characterization. Biochemistry. 1974 Aug 13;13(17):3487–3493. doi: 10.1021/bi00714a011. [DOI] [PubMed] [Google Scholar]
- Tosteson T. R., Corpe W. A. Enhancement of adhesion of the marine Chlorella vulgaris to glass. Can J Microbiol. 1975 Jul;21(7):1025–1031. doi: 10.1139/m75-152. [DOI] [PubMed] [Google Scholar]



