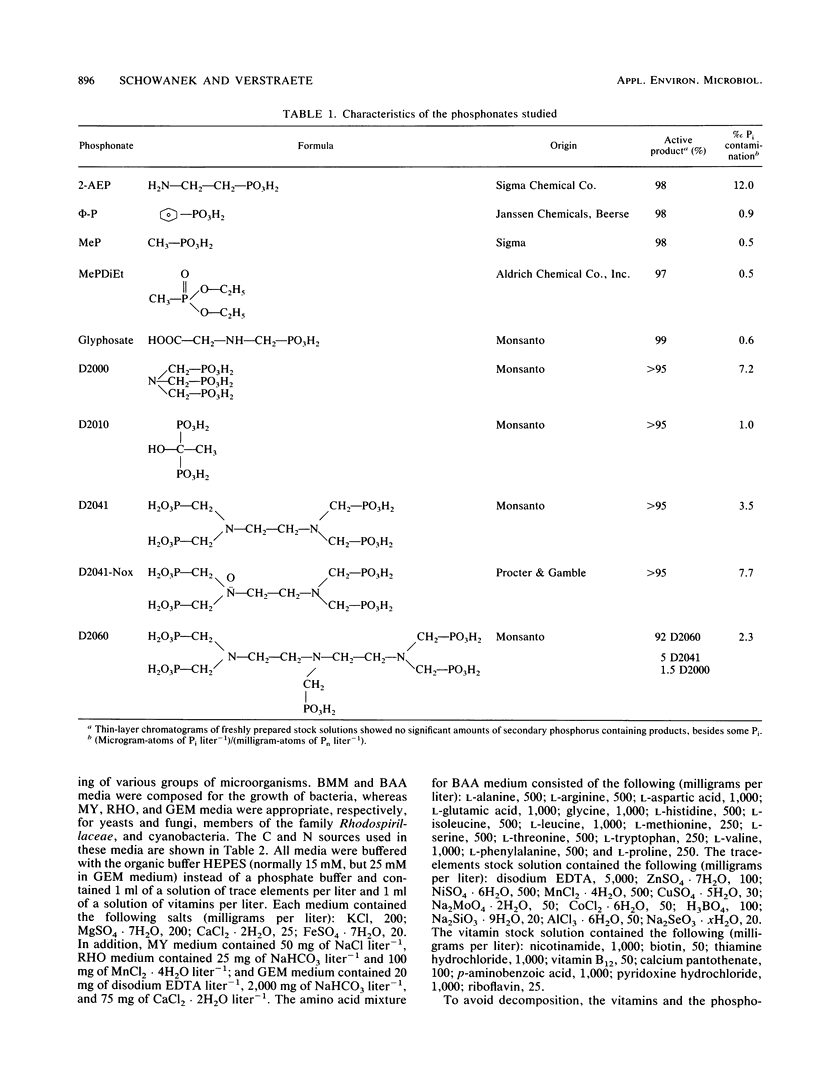
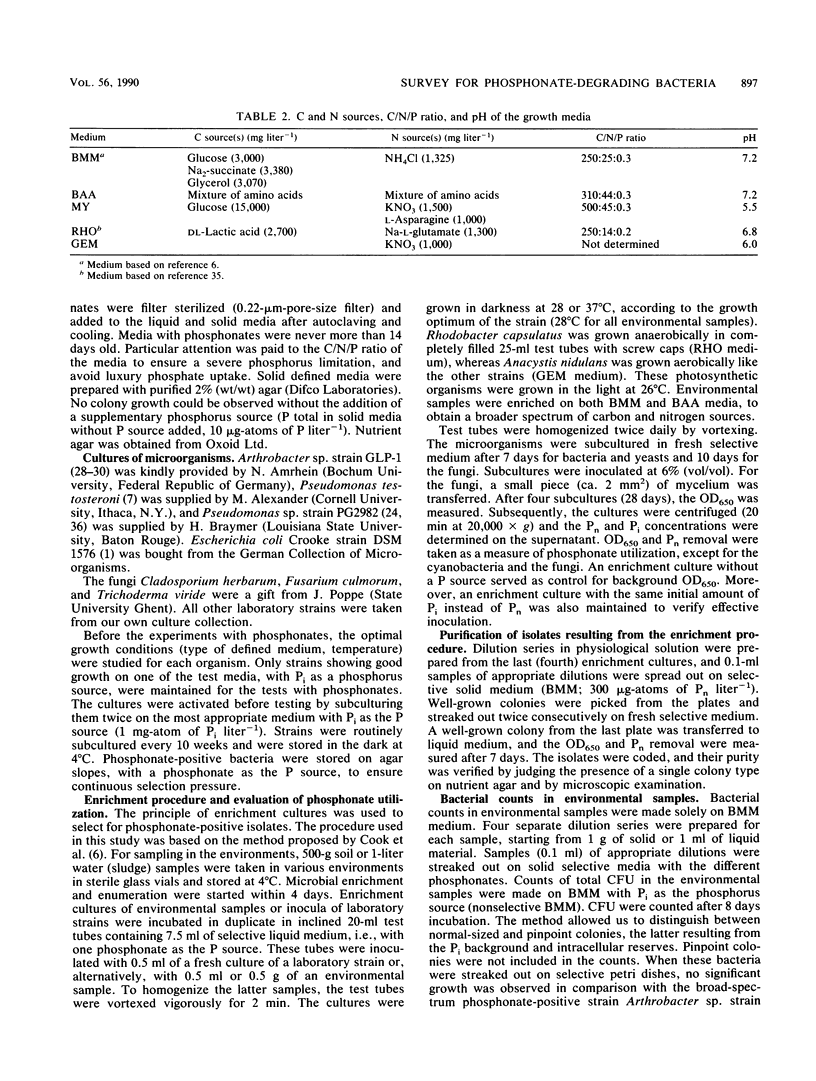
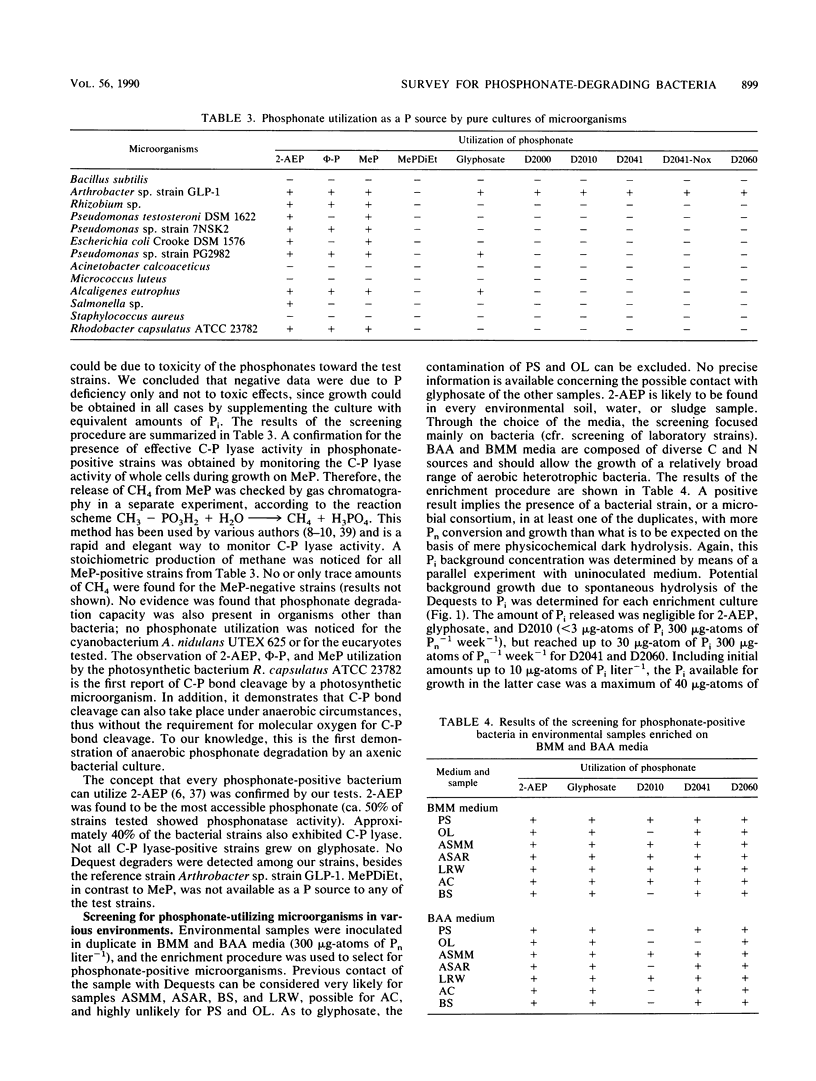
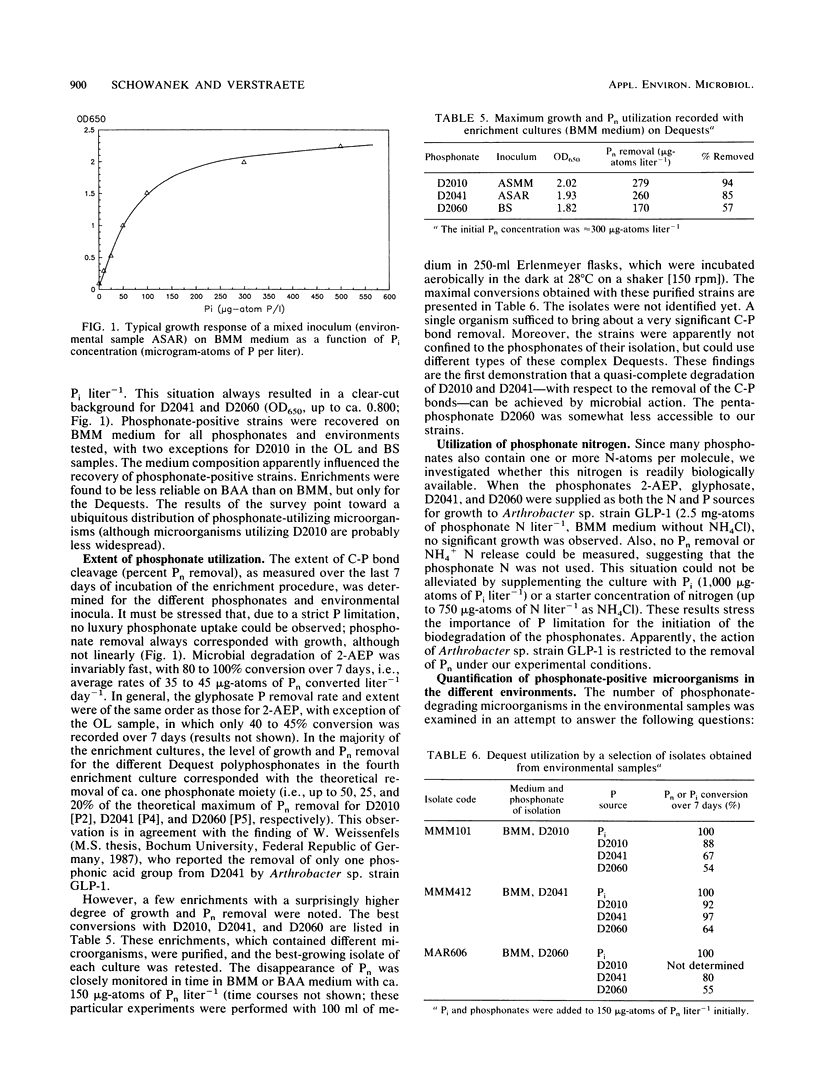
Abstract
A selection of axenic microbial strains and a variety of environmental samples were investigated with respect to the utilization of a series of natural and xenobiotic phosphonates as the sole phosphorus source for growth. Phosphonate degradation was observed only with bacteria and not with eucaryotic microorganisms. All representatives of the phosphonates examined supported bacterial growth, with the exception of methylphosphonate diethylester. Yet, distinctly different phosphonate utilization patterns were noted between phosphonate-positive strains. C-P bond cleavage by a photosynthetic bacterium is reported for the first time; growing photoheterotrophically, Rhodobacter capsulatus ATCC 23782 was able to utilize 2-aminoethylphosphonate and alkylphosphonates. Bacteria with the potential to utilize at least one of the phosphonate moieties from the xenobiotic phosphonates Dequest 2010, Dequest 2041, and Dequest 2060 were detected in all environments, with only two exceptions for Dequest 2010. Phosphonate P utilization to an extent of 94 and 97%, for Dequest 2010 and Dequest 2041, respectively, provided evidence that a complete breakdown of these compounds with respect to the C-P bond cleavage can be achieved by some bacteria. The results suggest that phosphonate-utilizing bacteria are ubiquitous, and that selected strains can degrade phosphonates that are more complex than those described previously.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alam A. U., Bishop S. H. Growth of Escherichia coli on some organophosphonic acids. Can J Microbiol. 1969 Sep;15(9):1043–1046. doi: 10.1139/m69-185. [DOI] [PubMed] [Google Scholar]
- Anderson D. J., Day M. J., Russell N. J., White G. F. Temporal and geographical distributions of epilithic sodium dodecyl sulfate-degrading bacteria in a polluted South Wales river. Appl Environ Microbiol. 1988 Feb;54(2):555–560. doi: 10.1128/aem.54.2.555-560.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthazor T. M., Hallas L. E. Glyphosate-degrading microorganisms from industrial activated sludge. Appl Environ Microbiol. 1986 Feb;51(2):432–434. doi: 10.1128/aem.51.2.432-434.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumpus J. A., Brock B. J. Biodegradation of crystal violet by the white rot fungus Phanerochaete chrysosporium. Appl Environ Microbiol. 1988 May;54(5):1143–1150. doi: 10.1128/aem.54.5.1143-1150.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Daughton C. G., Alexander M. Phosphonate utilization by bacteria. J Bacteriol. 1978 Jan;133(1):85–90. doi: 10.1128/jb.133.1.85-90.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A. M., Daughton C. G., Alexander M. Phosphorus-containing pesticide breakdown products: quantitative utilization as phosphorus sources by bacteria. Appl Environ Microbiol. 1978 Nov;36(5):668–672. doi: 10.1128/aem.36.5.668-672.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daughton C. G., Cook A. M., Alexander M. Gas chromatographic determination of phosphorus-containing pesticide metabolites via benzylation. Anal Chem. 1979 Oct;51(12):1949–1953. doi: 10.1021/ac50048a014. [DOI] [PubMed] [Google Scholar]
- Egli T. (An)aerobic breakdown of chelating agents used in household detergents. Microbiol Sci. 1988 Feb;5(2):36–41. [PubMed] [Google Scholar]
- Fitzgibbon J., Braymer H. D. Phosphate starvation induces uptake of glyphosate by Pseudomonas sp. strain PG2982. Appl Environ Microbiol. 1988 Jul;54(7):1886–1888. doi: 10.1128/aem.54.7.1886-1888.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkness D. R. Bacterial growth on aminoalkylphosphonic acids. J Bacteriol. 1966 Sep;92(3):623–627. doi: 10.1128/jb.92.3.623-627.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- La Nauze J. M., Rosenberg H., Shaw D. C. The enzymic cleavage of the carbon-phosphorus bond: purification and properties of phosphonatase. Biochim Biophys Acta. 1970 Aug 15;212(2):332–350. doi: 10.1016/0005-2744(70)90214-7. [DOI] [PubMed] [Google Scholar]
- Lund-Høie K., Friestad H. O. Photodegradation of the herbicide glyphosate in water. Bull Environ Contam Toxicol. 1986 May;36(5):723–729. doi: 10.1007/BF01623575. [DOI] [PubMed] [Google Scholar]
- Moore J. K., Braymer H. D., Larson A. D. Isolation of a Pseudomonas sp. Which Utilizes the Phosphonate Herbicide Glyphosate. Appl Environ Microbiol. 1983 Aug;46(2):316–320. doi: 10.1128/aem.46.2.316-320.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overbeeke N., Lugtenberg B. Recognition site for phosphorus-containing compounds and other negatively charged solutes on the PhoE protein pore of the outer membrane of Escherichia coli K12. Eur J Biochem. 1982 Aug;126(1):113–118. doi: 10.1111/j.1432-1033.1982.tb06754.x. [DOI] [PubMed] [Google Scholar]
- Pipke R., Amrhein N. Degradation of the Phosphonate Herbicide Glyphosate by Arthrobacter atrocyaneus ATCC 13752. Appl Environ Microbiol. 1988 May;54(5):1293–1296. doi: 10.1128/aem.54.5.1293-1296.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipke R., Amrhein N. Isolation and Characterization of a Mutant of Arthrobacter sp. Strain GLP-1 Which Utilizes the Herbicide Glyphosate as Its Sole Source of Phosphorus and Nitrogen. Appl Environ Microbiol. 1988 Nov;54(11):2868–2870. doi: 10.1128/aem.54.11.2868-2870.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pipke R., Amrhein N., Jacob G. S., Schaefer J., Kishore G. M. Metabolism of glyphosate in an Arthrobacter sp. GLP-1. Eur J Biochem. 1987 Jun 1;165(2):267–273. doi: 10.1111/j.1432-1033.1987.tb11437.x. [DOI] [PubMed] [Google Scholar]
- Porra R. J., Grimme L. H. A new procedure for the determination of chlorophylls a and b and its application to normal and regreening Chlorella. Anal Biochem. 1974 Jan;57(1):255–267. doi: 10.1016/0003-2697(74)90071-2. [DOI] [PubMed] [Google Scholar]
- Rosenberg H., La Nauze J. M. The metabolism of phosphonates by microorganisms. The transport of aminoethylphosphonic acid in Bacillus cereus. Biochim Biophys Acta. 1967 Jun 13;141(1):79–90. doi: 10.1016/0304-4165(67)90247-4. [DOI] [PubMed] [Google Scholar]
- Rueppel M. L., Brightwell B. B., Schaefer J., Marvel J. T. Metabolism and degradation of glyphosphate in soil and water. J Agric Food Chem. 1977 May-Jun;25(3):517–528. doi: 10.1021/jf60211a018. [DOI] [PubMed] [Google Scholar]
- Shinabarger D. L., Schmitt E. K., Braymer H. D., Larson A. D. Phosphonate Utilization by the Glyphosate-Degrading Pseudomonas sp. Strain PG2982. Appl Environ Microbiol. 1984 Nov;48(5):1049–1050. doi: 10.1128/aem.48.5.1049-1050.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Shames S. L., Venditti C. P., Walsh C. T. Bacterial carbon-phosphorus lyase: products, rates, and regulation of phosphonic and phosphinic acid metabolism. J Bacteriol. 1987 Feb;169(2):710–717. doi: 10.1128/jb.169.2.710-717.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wackett L. P., Wanner B. L., Venditti C. P., Walsh C. T. Involvement of the phosphate regulon and the psiD locus in carbon-phosphorus lyase activity of Escherichia coli K-12. J Bacteriol. 1987 Apr;169(4):1753–1756. doi: 10.1128/jb.169.4.1753-1756.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


