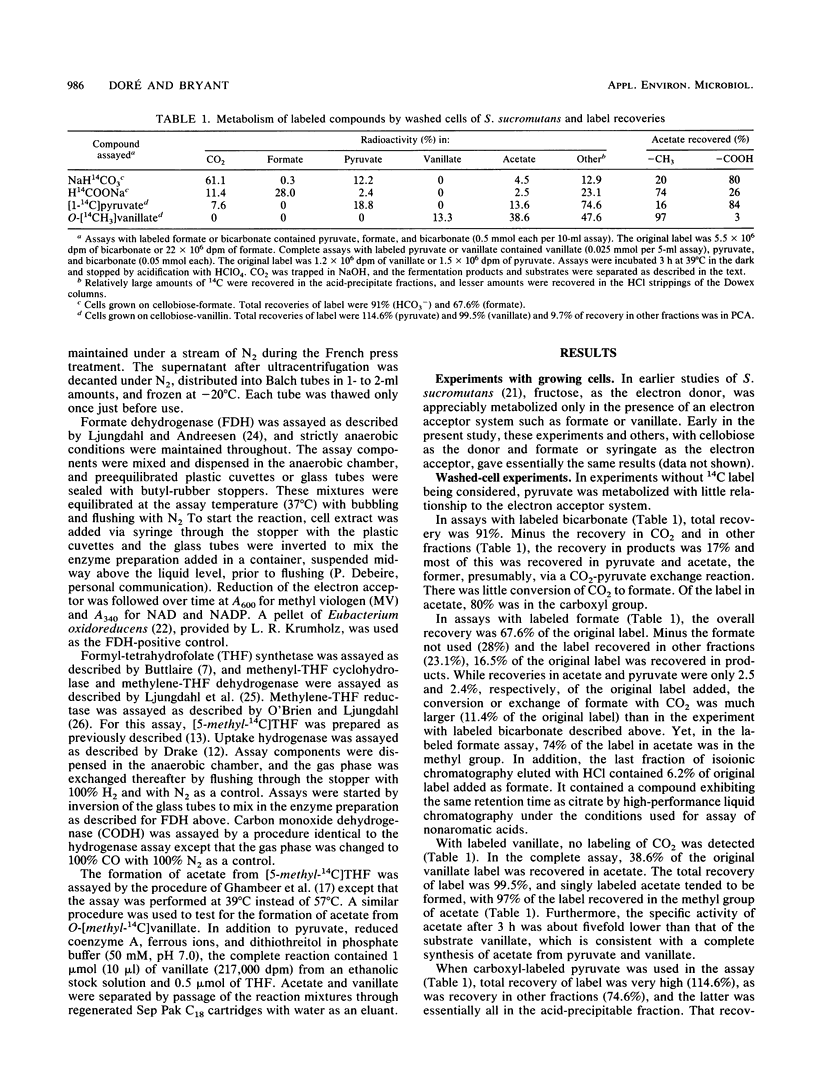
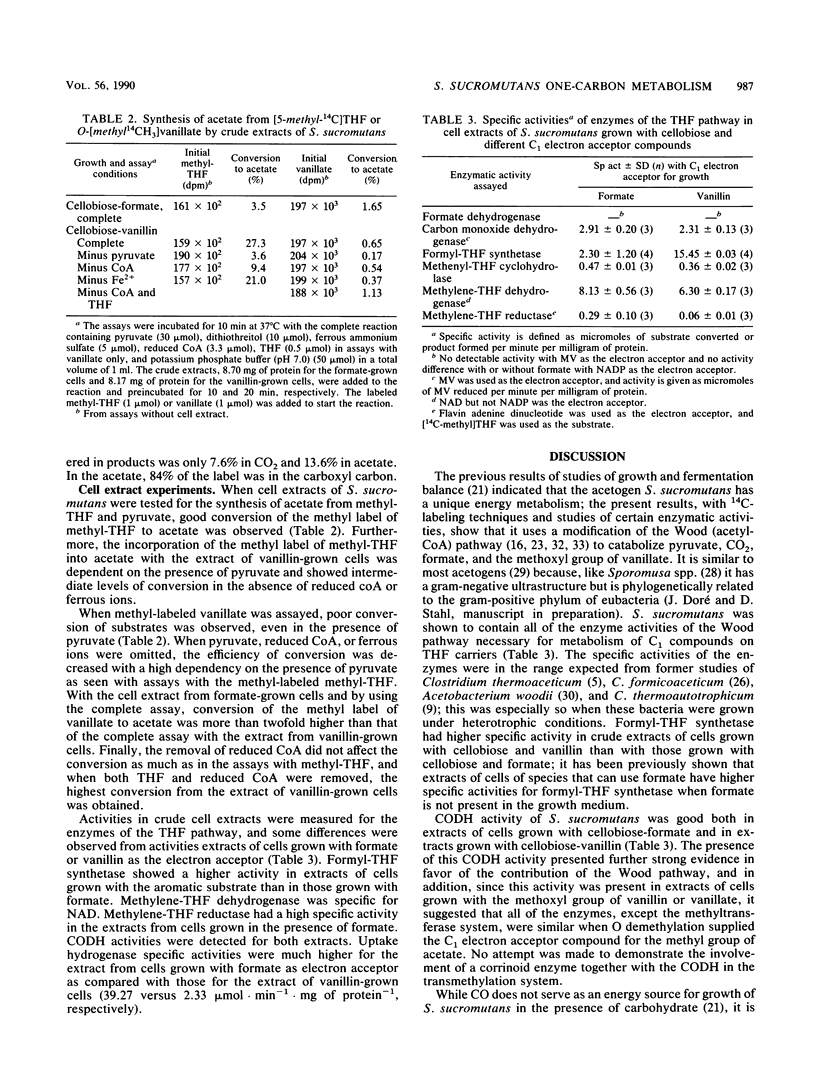
Abstract
Syntrophococcus sucromutans is the predominant species capable of O demethylation of methoxylated lignin monoaromatic derivatives in the rumen. The enzymatic characterization of this acetogen indicated that it uses the acetyl coenzyme A (Wood) pathway. Cell extracts possess all the enzymes of the tetrahydrofolate pathway, as well as carbon monoxide dehydrogenase, at levels similar to those of other acetogens using this pathway. However, formate dehydrogenase could not be detected in cell extracts, whether formate or a methoxyaromatic was used as electron acceptor for growth of the cells on cellobiose. Labeled bicarbonate, formate, [1-14C] pyruvate, and chemically synthesized O-[methyl-14C]vanillate were used to further investigate the catabolism of one-carbon (C1) compounds by using washed-cell preparations. The results were consistent with little or no contribution of formate dehydrogenase and pointed out some unique features. Conversion of formate to CO2 was detected, but labeled formate predominantly labeled the methyl group of acetate. Labeled CO2 readily exchanged with the carboxyl group of pyruvate but not with formate, and both labeled CO2 and pyruvate predominantly labeled the carboxyl group of acetate. No CO2 was formed from O demethylation of vanillate, and the acetate produced was position labeled in the methyl group. The fermentation pattern and specific activities of products indicated a complete synthesis of acetate from pyruvate and the methoxyl group of vanillate.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., El Ghazzawi E., Gottschalk G. The effect of ferrous ions, tungstate and selenite on the level of formate dehydrogenase in Clostridium formicoaceticum and formate synthesis from CO2 during pyruvate fermentation. Arch Mikrobiol. 1974 Mar 4;96(2):103–118. doi: 10.1007/BF00590167. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Gottschalk G., Schlegel H. G. Clostridium formicoaceticum nov. spec. isolation, description and distinction from C. aceticum and C. thermoaceticum. Arch Mikrobiol. 1970;72(2):154–174. doi: 10.1007/BF00409521. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Ljungdahl L. G. Formate dehydrogenase of Clostridium thermoaceticum: incorporation of selenium-75, and the effects of selenite, molybdate, and tungstate on the enzyme. J Bacteriol. 1973 Nov;116(2):867–873. doi: 10.1128/jb.116.2.867-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttlaire D. H. Purification and properties of formyltetrahydrofolate synthetase. Methods Enzymol. 1980;66:585–599. doi: 10.1016/0076-6879(80)66512-4. [DOI] [PubMed] [Google Scholar]
- Clark J. E., Ragsdale S. W., Ljungdahl L. G., Wiegel J. Levels of enzymes involved in the synthesis of acetate from CO2 in Clostridium thermoautotrophicum. J Bacteriol. 1982 Jul;151(1):507–509. doi: 10.1128/jb.151.1.507-509.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doré J., Bryant M. P. Lipid growth requirement and influence of lipid supplement on fatty acid and aldehyde composition of Syntrophococcus sucromutans. Appl Environ Microbiol. 1989 Apr;55(4):927–933. doi: 10.1128/aem.55.4.927-933.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake H. L., Hu S. I., Wood H. G. Purification of five components from Clostridium thermoaceticum which catalyze synthesis of acetate from pyruvate and methyltetrahydrofolate. Properties of phosphotransacetylase. J Biol Chem. 1981 Nov 10;256(21):11137–11144. [PubMed] [Google Scholar]
- Drake H. L. Occurrence of nickel in carbon monoxide dehydrogenase from Clostridium pasteurianum and Clostridium thermoaceticum. J Bacteriol. 1982 Feb;149(2):561–566. doi: 10.1128/jb.149.2.561-566.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Bossert I., Young L. Y. Enzymatic aryl-o-methyl-C labeling of model lignin monomers. Appl Environ Microbiol. 1986 Jan;51(1):80–83. doi: 10.1128/aem.51.1.80-83.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frazer A. C., Young L. Y. Anaerobic c(1) metabolism of the o-methyl-C-labeled substituent of vanillate. Appl Environ Microbiol. 1986 Jan;51(1):84–87. doi: 10.1128/aem.51.1.84-87.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghambeer R. K., Wood H. G., Schulman M., Ljungdahl L. Total synthesis of acetate from CO2. 3. Inhibition by alkylhalides of the synthesis from CO2, methyltetrahydrofolate, and methyl-B12 by Clostridium thermoaceticum. Arch Biochem Biophys. 1971 Apr;143(2):471–484. doi: 10.1016/0003-9861(71)90232-3. [DOI] [PubMed] [Google Scholar]
- Hungate R. E., Smith W., Bauchop T., Yu I., Rabinowitz J. C. Formate as an intermediate in the bovine rumen fermentation. J Bacteriol. 1970 May;102(2):389–397. doi: 10.1128/jb.102.2.389-397.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBLATT J. A., BERNATH P., KATZ J. THE DETERMINATION OF SPECIFIC ACTIVITY OF BAC14O3 BY LIQUID SCINTILLATION ASSAY. Int J Appl Radiat Isot. 1964 Apr;15:191–194. doi: 10.1016/0020-708x(64)90065-1. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G., Andreesen J. R. Formate dehydrogenase, a selenium--tungsten enzyme from Clostridium thermoaceticum. Methods Enzymol. 1978;53:360–372. doi: 10.1016/s0076-6879(78)53042-5. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G., O'Brien W. E., Moore M. R., Liu M. T. Methylenetetrahydrofolate dehydrogenase from Clostridium formicoaceticum and methylenetetrahydrofolate dehydrogenase, methenyltetrahydrofolate cyclohydrolase (combined) from Clostridium thermoaceticum. Methods Enzymol. 1980;66:599–609. doi: 10.1016/0076-6879(80)66513-6. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L. G. The autotrophic pathway of acetate synthesis in acetogenic bacteria. Annu Rev Microbiol. 1986;40:415–450. doi: 10.1146/annurev.mi.40.100186.002215. [DOI] [PubMed] [Google Scholar]
- O'Brien W. E., Ljungdahl L. G. Fermentation of fructose and synthesis of acetate from carbon dioxide by Clostridium formicoaceticum. J Bacteriol. 1972 Feb;109(2):626–632. doi: 10.1128/jb.109.2.626-632.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHARES E. F. Degradation of labeled propionic and acetic acids. Arch Biochem Biophys. 1951 Sep;33(2):173–178. doi: 10.1016/0003-9861(51)90094-x. [DOI] [PubMed] [Google Scholar]
- Tanner R. S., Wolfe R. S., Ljungdahl L. G. Tetrahydrofolate enzyme levels in Acetobacterium woodii and their implication in the synthesis of acetate from CO2. J Bacteriol. 1978 May;134(2):668–670. doi: 10.1128/jb.134.2.668-670.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Rupprecht E., Jungermann K. Separation of 14C-formate from CO2 fixation metabolites by isoionic-exchange chromatography. Anal Biochem. 1970 Dec;38(2):461–468. doi: 10.1016/0003-2697(70)90471-9. [DOI] [PubMed] [Google Scholar]
- Wu Z. R., Daniel S. L., Drake H. L. Characterization of a CO-dependent O-demethylating enzyme system from the acetogen Clostridium thermoaceticum. J Bacteriol. 1988 Dec;170(12):5747–5750. doi: 10.1128/jb.170.12.5747-5750.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto I., Saiki T., Liu S. M., Ljungdahl L. G. Purification and properties of NADP-dependent formate dehydrogenase from Clostridium thermoaceticum, a tungsten-selenium-iron protein. J Biol Chem. 1983 Feb 10;258(3):1826–1832. [PubMed] [Google Scholar]



