Abstract
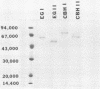
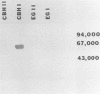
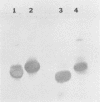
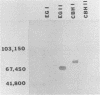
Splenocytes derived from mice inoculated with a commercial cellulase preparation or purified cellulases were fused with a stable myeloma cell line (SP2/0). Specific monoclonal antibodies to cellobiohydrolases I and II and endoglucanases I and II were established. In addition to specific monoclonal antibodies, we were also able to establish stable hybridoma cell lines which produced monoclonal antibodies that recognized similar epitopes possessed by two or more of the above cellulases. By obtaining monospecific antibodies for all four individual cellulases, the role and function of the individual cellulases can thus be studied in greater detail.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berghem L. E., Pettersson L. G., Axiö-Fredriksson U. B. The mechanism of enzymatic cellulose degradation. Purification and some properties of two different 1,4beta-glucan glucanohydrolases from Trichoderma viride. Eur J Biochem. 1976 Jan 15;61(2):621–630. doi: 10.1111/j.1432-1033.1976.tb10058.x. [DOI] [PubMed] [Google Scholar]
- Fägerstam L. G., Pettersson L. G. The cellulolytic complex of Trichoderma reesei QM 9414. An immunochemical approach. FEBS Lett. 1979 Feb 15;98(2):363–367. doi: 10.1016/0014-5793(79)80218-5. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Mandels M. Applications of cellulases. Biochem Soc Trans. 1985 Apr;13(2):414–416. doi: 10.1042/bst0130414. [DOI] [PubMed] [Google Scholar]
- Mischak H., Hofer F., Messner R., Weissinger E., Hayn M., Tomme P., Esterbauer H., Küchler E., Claeyssens M., Kubicek C. P. Monoclonal antibodies against different domains of cellobiohydrolase I and II from Trichoderma reesei. Biochim Biophys Acta. 1989 Jan 27;990(1):1–7. doi: 10.1016/s0304-4165(89)80003-0. [DOI] [PubMed] [Google Scholar]
- Nummi M., Niku-Paavola M. L., Enari T. M., Raunio V. Immunoelectrophoretic detection of cellulases. FEBS Lett. 1980 May 5;113(2):164–166. doi: 10.1016/0014-5793(80)80583-7. [DOI] [PubMed] [Google Scholar]
- Okada G. Enzymatic studies on a cellulase system of Trichoderma viride. IV. Purification and properties of a less-random type cellulase. J Biochem. 1976 Nov;80(5):913–922. doi: 10.1093/oxfordjournals.jbchem.a131377. [DOI] [PubMed] [Google Scholar]
- Penttilä M., Lehtovaara P., Nevalainen H., Bhikhabhai R., Knowles J. Homology between cellulase genes of Trichoderma reesei: complete nucleotide sequence of the endoglucanase I gene. Gene. 1986;45(3):253–263. doi: 10.1016/0378-1119(86)90023-5. [DOI] [PubMed] [Google Scholar]
- REESE E. T., SIU R. G. H., LEVINSON H. S. The biological degradation of soluble cellulose derivatives and its relationship to the mechanism of cellulose hydrolysis. J Bacteriol. 1950 Apr;59(4):485–497. doi: 10.1128/jb.59.4.485-497.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoemaker S. P., Brown R. D., Jr Characterization of endo-1,4-beta-D-glucanases purified from Trichoderma viride. Biochim Biophys Acta. 1978 Mar 14;523(1):147–161. doi: 10.1016/0005-2744(78)90017-7. [DOI] [PubMed] [Google Scholar]
- Shulman M., Wilde C. D., Köhler G. A better cell line for making hybridomas secreting specific antibodies. Nature. 1978 Nov 16;276(5685):269–270. doi: 10.1038/276269a0. [DOI] [PubMed] [Google Scholar]
- Teeri T. T., Lehtovaara P., Kauppinen S., Salovuori I., Knowles J. Homologous domains in Trichoderma reesei cellulolytic enzymes: gene sequence and expression of cellobiohydrolase II. Gene. 1987;51(1):43–52. doi: 10.1016/0378-1119(87)90472-0. [DOI] [PubMed] [Google Scholar]