Abstract
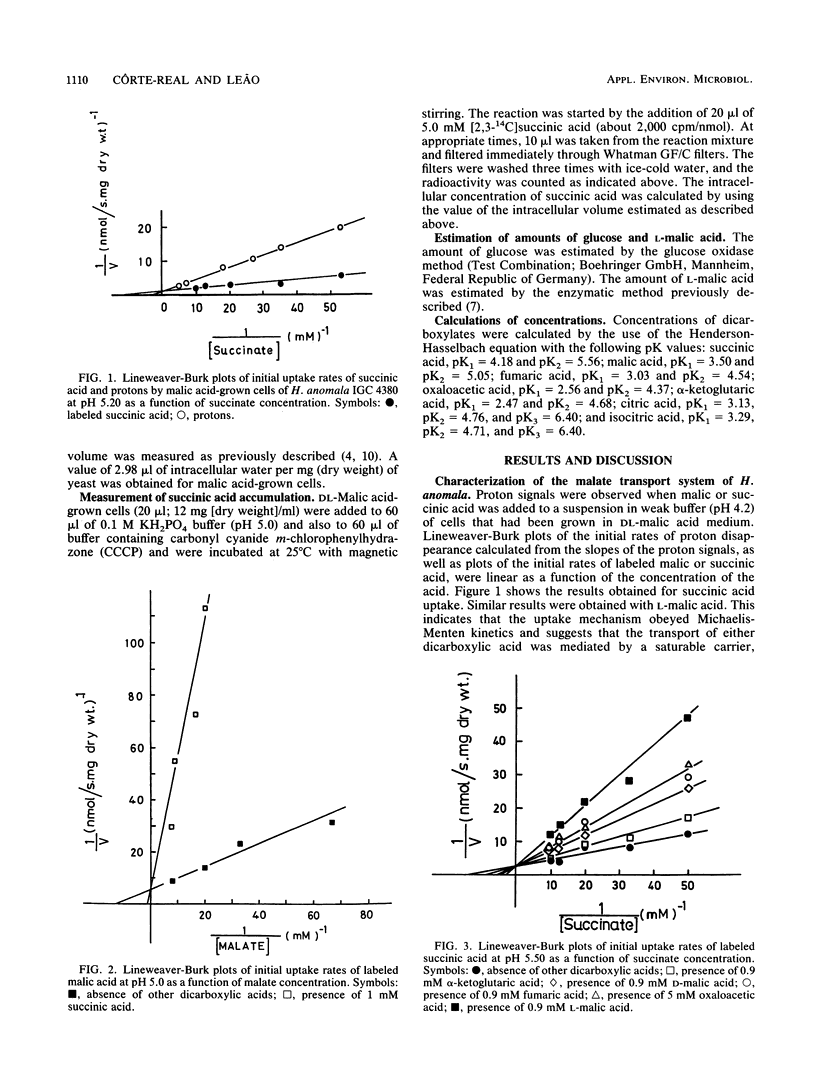
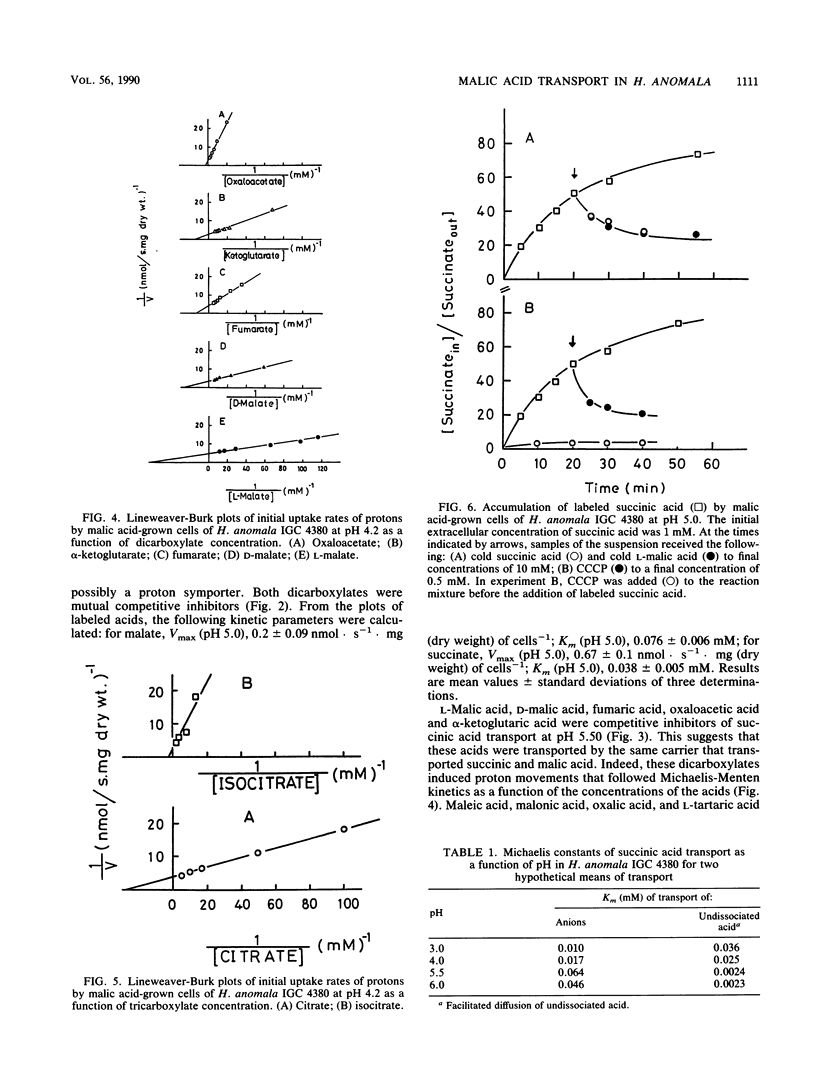
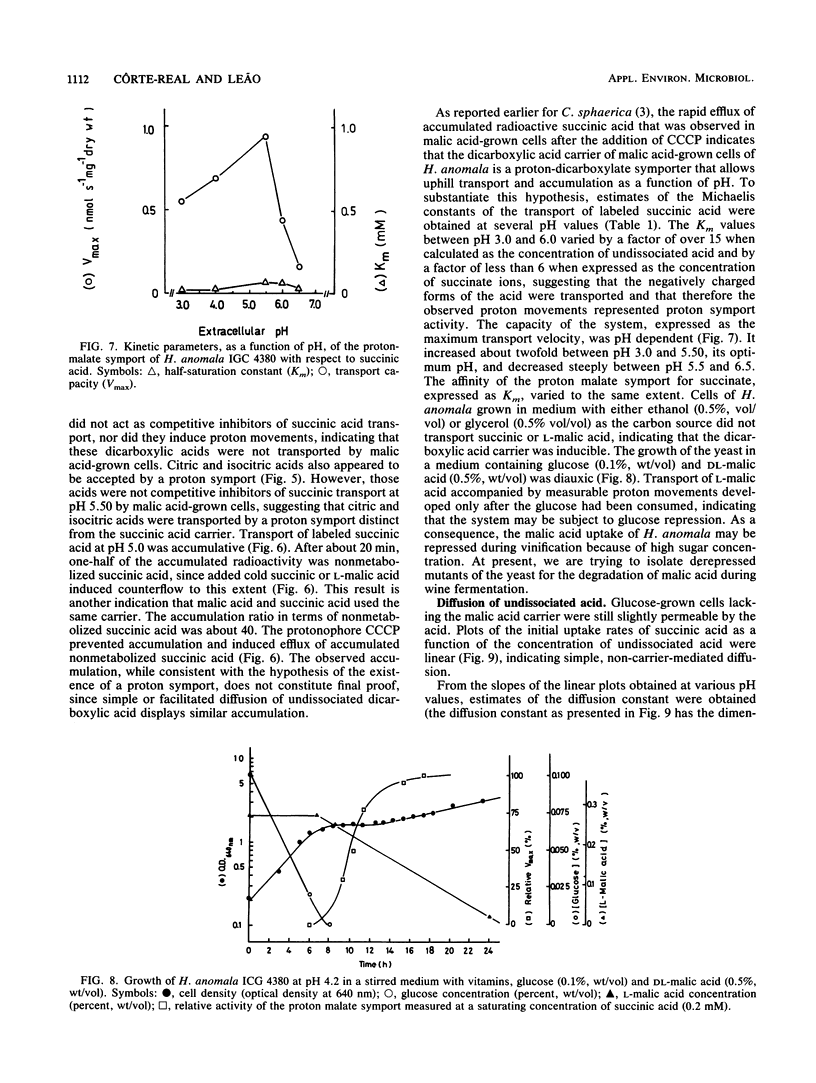
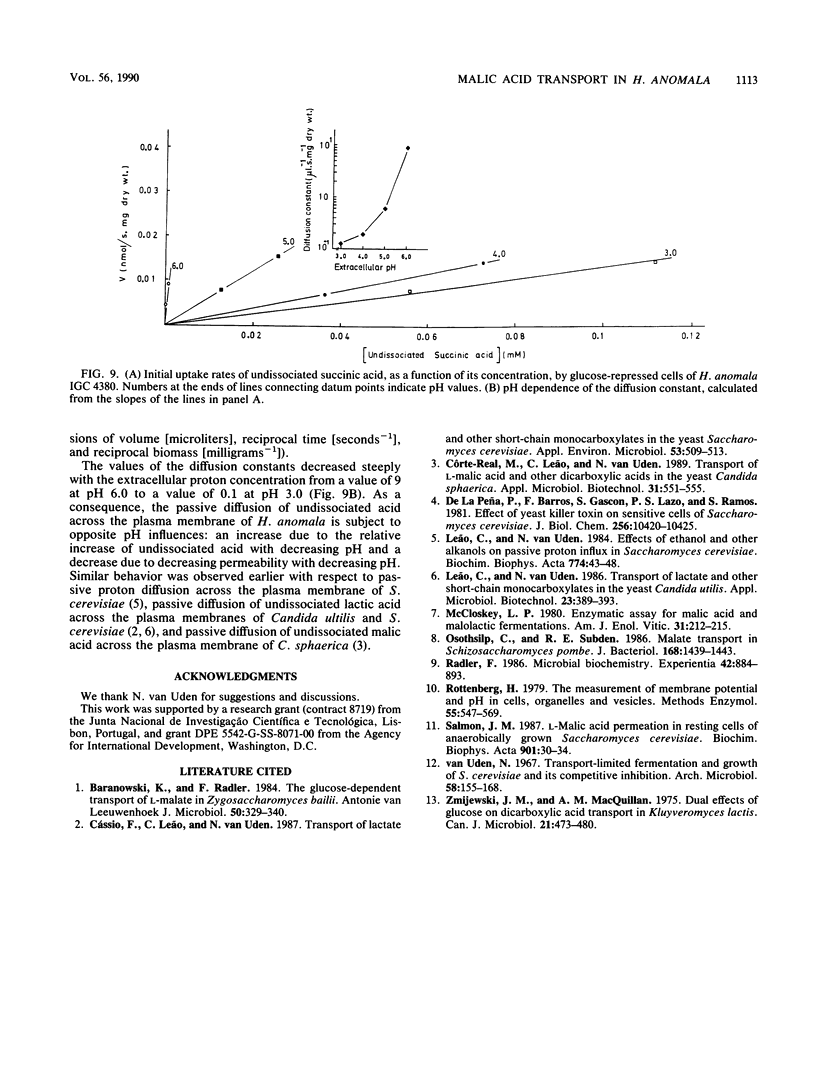
DL-Malic acid-grown cells of the yeast Hansenula anomala formed a saturable transport system that mediated accumulative transport of L-malic acid with the following kinetic parameters at pH 5.0: Vmax, 0.20 nmol.s-1.mg (dry weight)-1; Km, 0.076 mM L-malate. Uptake of malic acid was accompanied by proton disappearance from the external medium with rates that followed Michaelis-Menten kinetics as a function of malic acid concentration. Fumaric acid, alpha-ketoglutaric acid, oxaloacetic acid, D-malic acid, and L-malic acid were competitive inhibitors of succinic acid transport, and all induced proton movements that followed Michaelis-Menten kinetics, suggesting that all of these dicarboxylates used the same transport system. Maleic acid, malonic acid, oxalic acid, and L-(+)-tartaric acid, as well as other Krebs cycle acids such as citric and isocitric acids, were not accepted by the malate transport system. Km measurements as a function of pH suggested that the anionic forms of the acids were transported by an accumulative dicarboxylate proton symporter. The accumulation ratio at pH 5.0 was about 40. The malate system was inducible and was subject to glucose repression. Undissociated succinic acid entered the cells slowly by simple diffusion. The permeability of the cells by undissociated acid increased with pH, with the diffusion constant increasing 100-fold between pH 3.0 and 6.0.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baranowski K., Radler F. The glucose-dependent transport of L-malate in Zygosaccharomyces bailii. Antonie Van Leeuwenhoek. 1984;50(4):329–340. doi: 10.1007/BF00394646. [DOI] [PubMed] [Google Scholar]
- Cássio F., Leão C., van Uden N. Transport of lactate and other short-chain monocarboxylates in the yeast Saccharomyces cerevisiae. Appl Environ Microbiol. 1987 Mar;53(3):509–513. doi: 10.1128/aem.53.3.509-513.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leão C., Van Uden N. Effects of ethanol and other alkanols on passive proton influx in the yeast Saccharomyces cerevisiae. Biochim Biophys Acta. 1984 Jul 11;774(1):43–48. doi: 10.1016/0005-2736(84)90272-4. [DOI] [PubMed] [Google Scholar]
- Osothsilp C., Subden R. E. Malate transport in Schizosaccharomyces pombe. J Bacteriol. 1986 Dec;168(3):1439–1443. doi: 10.1128/jb.168.3.1439-1443.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottenberg H. The measurement of membrane potential and deltapH in cells, organelles, and vesicles. Methods Enzymol. 1979;55:547–569. doi: 10.1016/0076-6879(79)55066-6. [DOI] [PubMed] [Google Scholar]
- Salmon J. M. L-malic-acid permeation in resting cells of anaerobically grown Saccharomyces cerevisiae. Biochim Biophys Acta. 1987 Jul 10;901(1):30–34. doi: 10.1016/0005-2736(87)90253-7. [DOI] [PubMed] [Google Scholar]
- Zmijewski M. J., Jr, MacQuillan A. M. Dual effects of glucose on dicarboxylic acid transport in Kluyveromyces lactis. Can J Microbiol. 1975 Apr;21(4):473–480. doi: 10.1139/m75-066. [DOI] [PubMed] [Google Scholar]
- de la Peña P., Barros F., Gascón S., Lazo P. S., Ramos S. Effect of yeast killer toxin on sensitive cells of Saccharomyces cerevisiae. J Biol Chem. 1981 Oct 25;256(20):10420–10425. [PubMed] [Google Scholar]
- van Uden N. Transport-limited fermentation and growth of saccharomyces cerevisiae and its competitive inhibition. Arch Mikrobiol. 1967;58(2):155–168. doi: 10.1007/BF00406676. [DOI] [PubMed] [Google Scholar]


