Abstract
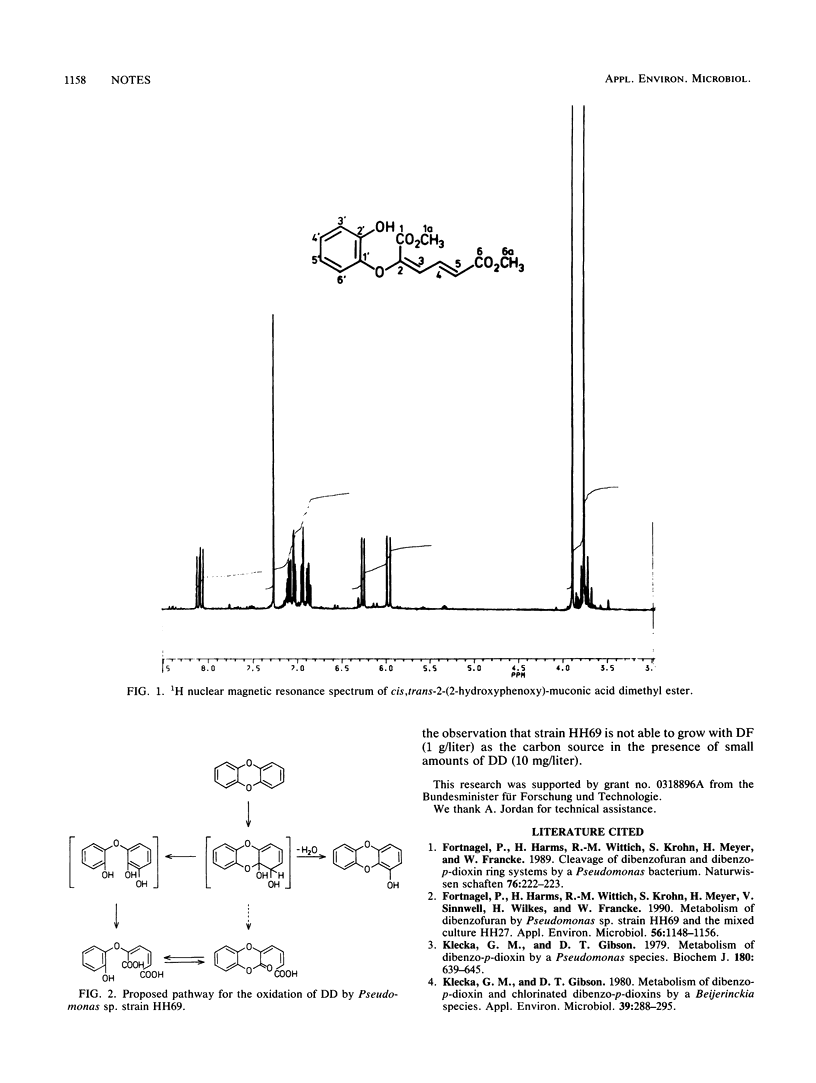
Dibenzo-p-dioxin was oxidatively cleaved by the dibenzofuran-degrading bacterium Pseudomonas sp. strain HH69 to produce minor amounts of 1-hydroxydibenzo-p-dioxin and catechol, while a 2-phenoxy derivative of muconic acid was formed as the major product. Upon acidic methylation, the latter yielded the dimethylester of cis, trans-2-(2-hydroxyphenoxy)-muconic acid.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fortnagel P., Harms H., Wittich R. M., Francke W., Krohn S., Meyer H. Cleavage of dibenzofuran and dibenzodioxin ring systems by a Pseudomonas bacterium. Naturwissenschaften. 1989 May;76(5):222–223. doi: 10.1007/BF00627694. [DOI] [PubMed] [Google Scholar]
- Fortnagel P., Harms H., Wittich R. M., Krohn S., Meyer H., Sinnwell V., Wilkes H., Francke W. Metabolism of Dibenzofuran by Pseudomonas sp. Strain HH69 and the Mixed Culture HH27. Appl Environ Microbiol. 1990 Apr;56(4):1148–1156. doi: 10.1128/aem.56.4.1148-1156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Metabolism of Dibenzo-p-Dioxin and Chlorinated Dibenzo-p- Dioxins by a Beijerinckia Species. Appl Environ Microbiol. 1980 Feb;39(2):288–296. doi: 10.1128/aem.39.2.288-296.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klecka G. M., Gibson D. T. Metabolism of dibenzo[1,4]dioxan by a Pseudomonas species. Biochem J. 1979 Jun 15;180(3):639–645. doi: 10.1042/bj1800639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. T., Matsumura F. Fate of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in a model aquatic environment. Arch Environ Contam Toxicol. 1978;7(3):349–357. doi: 10.1007/BF02332062. [DOI] [PubMed] [Google Scholar]



