Abstract
Visual development is thought to be completed at an early age. We suggest that the maturation of the visual brain is not homogeneous: functions with greater need for early availability, such as visuomotor control, mature earlier, and the development of other visual functions may extend well into childhood. We found significant improvement in children between 5 and 14 years in visual spatial integration by using a contour-detection task. The data show that long-range spatial interactions—subserving the integration of orientational information across the visual field—span a shorter spatial range in children than in adults. Performance in the task improves in a cue-specific manner with practice, which indicates the participation of fairly low-level perceptual mechanisms. We interpret our findings in terms of a protracted development of ventral visual-stream function in humans.
Human visual development has been considered to be relatively fast and to give way to cognitive development after the basic visual functions are established in infancy, e.g., a very early preference for moving stimuli (1); the ability to process complex motion information at 4 months (2); color (3) and depth (4) discrimination also at around 4 months; and rapidly increasing acuity during the first year (5). However, human anatomical data indicate that, although the gross anatomical structure is constructed before birth, the maturation of neuronal circuits of the visual cortex may extend well into childhood (6, 7). More recent studies even raise the possibility of a significant increase in the number of cortical cells between birth and 6 years of age (8), implying a strikingly extended structural maturation of the human cortex, including the early visual areas (9). In light of these results, the question arises as to whether the maturation of human vision really comes to an end by the first or second year of life.
Although behavioral studies of human visual development beyond the second year of age are rare, there is indication that children may encounter problems in tasks involving integration of information across the visual field for object representation: visual segmentation and form identification based on contrasts in texture (10, 11), motion (12), or color (13) and recognition of incomplete objects (14). Here, we directly test the development of visual spatial integration in a contour-detection task. We find that children (aged 5–14 years) perform poorly in the task compared with adults. Our control results clearly show that perceptual immaturity lies behind the poor performance. The results also suggest that there is immaturity at the level of long-range spatial interactions that might span a shorter spatial range in children than in adults.
Experiment 1: Human Development of Spatial Integration
To segment the visual image and to form object boundaries in the course of perceptual organization, local orientational information extracted by selectively tuned neurons has to be integrated across the visual field. The efficiency of the integrating mechanism can be estimated psychophysically in a contour-detection task that employs orientational noise (15–18). To study the human developmental pattern of spatial integration, we used a card-test version (19–20) of such a contour-detection task. The card version was suitable for young children, and it also allowed us to test a large number of subjects. As illustrated in Fig. 1a, the cards consisted of a closed chain of colinearly aligned Gabor signals (contour) and a background of randomly oriented and positioned Gabor signals (noise). Gabor signals roughly model the receptive field properties of orientation-selective simple cells in the primary visual cortex (V1). Therefore, Gabor signals are appropriate stimuli for the examination of these small spatial filters and their interactions. Notice that the contours cannot be detected purely by local filters or by neurons with large receptive field sizes corresponding to the size of the contour. The long-range orientational correlations along the path of the contour can be found only by the integration of local orientational measurements. The noise forces the observer to do these local measurements at the scale of the individual Gabor signals and to rely solely on long-range interactions between local filters while connecting the signals perceptually. Thus, these cards let us isolate the long-range interactions subserving spatial integration.
Figure 1.
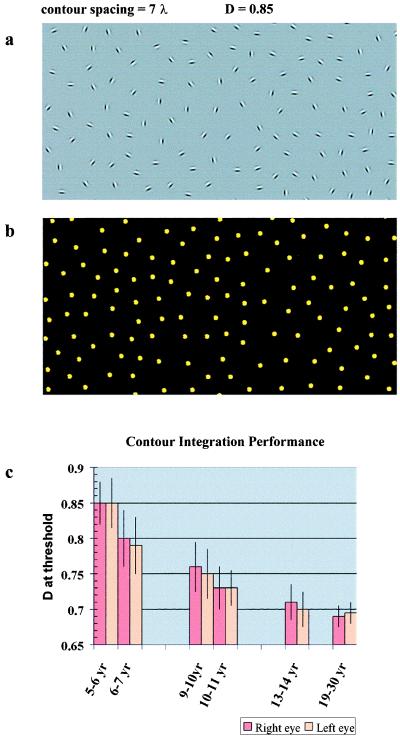
(a) An example of the contour-integration cards that are employed to study spatial integration. There is a closed figure embedded among the randomly positioned and oriented distractors. The subject’s task is to find the path of the contour. The difficulty level of each card is determined by the relative density of noise elements and is expressed as a ratio of average noise spacing over contour spacing (D). D = 0.85 in the present example. Absolute contour spacing is expressed in Gabor wavelength units (λ). The strength of spatial interactions subserving contour integration in an individual is indicated by the value of D at their threshold. The contour-integration cards were generated on a Silicon Graphics Indy R4000 computer (Mountain View, CA). The orientation-defined cards were then printed on a 2,400-dots-per-inch printer, and the color-defined cards (see Fig. 3c) were printed on an Epson Stylus Color 800 printer. Carrier frequency of the Gabor patches was 5 cycles per degree at a 57-cm viewing distance, and their contrast was about 95%. The luminance contrast and the size of the colored patches were randomized to ensure that the contour is purely defined by chromatic contrast and not by luminance contrast. Commission Internationale de l‘Eclairage chromaticity coordinates of the red dots ranged between x = 0.385, y = 0.274 and x = 0.509, y = 0.484, and the green dots ranged between x = 0.240, y = 0.301 and x = 0.550, y = 0.604. Mean luminance depended on the illumination in the room. Spacing between elements along the contour and spacing in the background were controlled independently. At small signal-to-noise ratios, background elements were allowed to get into the spaces between contour elements, but orientational alignment was avoided. The algorithm allowed us to keep the smallest permitted separation between background elements, while avoiding spurious spacings. A different random shape and background were computed for each card. The length of the contours was constant, and the contours had a continuously positive curvature with no inflection points. (b) The contour cannot be perceived when orientational information is removed. The Gabor signals shown in a are replaced by the dots, and the card is turned around to avoid the localization of the contour based on a. Information about the position of each element is still available; however, the contour remains hidden, illustrating that the only useful information in a is good continuity of oriented contour elements. (c) Contour integration performance as a function of age at six different age points. Performance is expressed by the average value of D at threshold for each age group. The performances of left and right eyes are shown separately.
We tested 510 subjects (97 adults and 413 children; 219 males and 291 females) with normal vision. The children ranged in age from 5 to 14 years with approximately the same number of children in five age groups. Subjects were recruited by advertisement, and the experiments were carried out at day-care centers, schools, and colleges in Szeged, Hungary. Before testing their contour-integration skills, the stereovision of the observers was tested with the Randot test, and their visual acuity was tested with E cards or Snellen cards depending on their age. Those with visual disorder, e.g., strabismus and amblyopia (2–5 subjects in each age group; 18 subjects in the entire sample), and those with a momentary inability to cooperate with the experimenter (5 in the entire sample) were excluded from the study. All included subjects had normal or corrected-to-normal visual acuity. Their two eyes were tested independently, which further excluded subjects with a possibility of amblyopia (contour-detection performance might be impaired and imbalanced in the two eyes of amblyopes; refs. 19, 20, 31). There was no significant effect of eye and gender with respect to contour-detection performance.
To estimate the actual strength of long-range interactions in each observer, we varied the relative noise density, while keeping contour spacing constant. Relative noise density (D) is defined as the ratio of average noise spacing over contour spacing. In our main study, we used a set of 10 cards in which D ranged between 1.1 and 0.65 and was varied with a step size of 0.05. When D > 1, the contour can be detected by using element-density information, because the contour elements are closer to each other than the noise elements. However, when D ≤ 1, this cue is not available, and it is impossible to detect the contour without orientation-specific long-range interactions (see Fig. 1b). The value of D at threshold (Dmin) defines the strength of the long-range interactions in an individual observer. The smaller these numbers are the stronger the interactions.
The cards were presented at about a 0.5-m distance. The subjects’ task was to identify the location of the contour and the trace of it within each card by pointing to the center of each closed contour and then following the path of the contour with their finger. Subjects were not forced to guess if they could not find the contour. The cards were presented in an increasing order of difficulty. Each card was presented only once in one session. One suprathreshold card was used as an explanatory example before the test. We determined Dmin—the value of D in the last correctly identified card—in one session for each observer for the right and left eyes separately.
As presented in Fig. 1c, children in the 13- to 14-year-old group were able to see most of the contours in the set (Dmin = 0.7), whereas the 5- to 6-year-old children missed the contours in about half of the cards (Dmin = 0.84). This difference in contour-integration performance between the two age groups is great (P < 0.005; two-tailed t test). Although the largest improvement seems to occur between the 5- to 6-year-old and the 6- to 7-year-old (Dmin = 0.79) groups, there is a tendency for gradually increasing performance in the other age groups as well: in the 9- to 10-year-old group, Dmin = 0.76; in the 10- to 11-year-old group, Dmin = 0.72. There is a slight improvement even after adolescence: in the 19- to 30-year-old group, Dmin = 0.67.
Why do young children perform poorly in the contour-integration task? To rule out the possibility that contrast sensitivity limits performance in the contour-detection task, we conducted a control study in which we looked at the performance of five normal adult observers as a function of the contrast of the Gabor signals. For better stimulus control, we used a computerized version of the task (15). Stimuli were presented on an Silicon Graphics Indy monitor, and stimulus presentation was controlled by a Silicon Graphics Indy R4000 computer. Mean luminance was 20 cd/m3. In a two-alternative forced-choice procedure, the observers had to indicate whether the contour was in the first- or second-presented frame (frame duration = 2 s). Using a staircase procedure, we estimated Dmin at different contrast values in separate blocks. We found saturated performance beyond 10–12% contrast. Children in our youngest age group (5–6 years) have at least 50% of the adult contrast sensitivity. Therefore, the results indicate that they are not limited by contrast sensitivity in the contour-detection task.
Experiment 2: Spatial Range of Interactions in Children and in Adults
We assumed that long-range orientation-specific spatial interactions are related to contour-integration performance and that the analysis of the actual spatial ranges of interactions in children and in adults might give some explanation of the developmental effect. These interactions might not be functioning at the adult level in terms of their spatial range. There exist psychophysical indications that the development of orientation-based segmentation in humans lasts longer than the development of luminance or motion-based segmentation (10, 11). There are also anatomical data showing that the intrinsic horizontal connections of the primary visual cortex (21, 22), which are assumed to provide the anatomical substance for long-range spatial interactions subserving contour integration and segmentation (23−25), develop at various times in the different layers of the primary visual cortex and seem to be immature even at 5 years of age in layer 2/3 in the human visual cortex (6). To see whether the spatial range of orientation-specific long-range interactions limits performance in children, we conducted an experiment in which we varied the spacing among contour elements, while keeping the relative noise level constant (see Fig. 2a).
Figure 2.
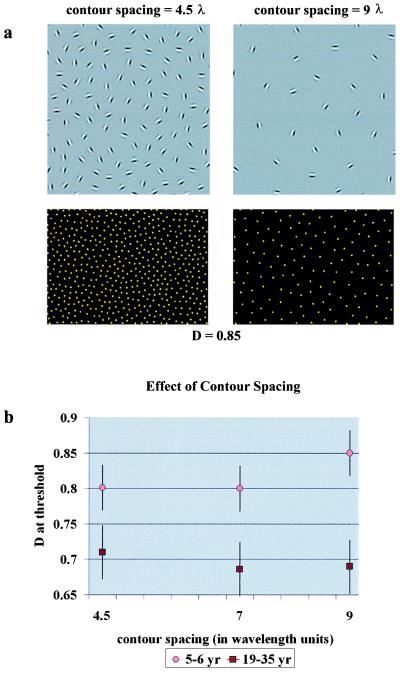
Analysis of the spatial ranges of interactions in children and in adults. Performance in the contour-integration task is determined by the relative noise density (D), and it might also be determined by the absolute (cortical) spatial range of interactions. We employed new sets of cards to see whether interaction ranges limit performance. (a) Examples of cards from the new sets. (Upper Left) Contour spacing is small: 4.5 λ. (Upper Right) Contour spacing is large: 9 λ. These are partial presentations of the cards showing only the contour area; for an intermediate spacing, see Fig. 1a. (Lower) The positions of all elements in the corresponding cards. D = 0.85 in both cases. (b) Child and adult contour-integration performances as a function of contour spacing. Adult performance as defined by D at threshold does not vary significantly in the tested contour-spacing range, which means that adults are limited only by relative noise density. However, children integrate large-spaced contours with a greater difficulty, which indicates the possibility of shorter interaction ranges in their case.
We determined Dmin for three different contour spacings (4.5, 7, and 9 λ) in adults (n = 54) and in 5- to 6-year-old children (n = 30). We used binocular testing, and we tested each child with the three sets of cards in one session. To eliminate the effect of practice, we used a counterbalanced design for the order of presentation of the three sets.
As Fig. 2b shows, Dmin in adults is independent of contour spacing. This independence indicates that they are limited only by display parameters (signal-to-noise ratio) in the tested range and not by the absolute range of cortical interactions (the range of contour spacings that can be tested at all is limited: above 9-λ spacing, the number of contour elements would be too small to provide comparable conditions). In children, however, we found better performance at smaller contour spacings. At 9-λ contour spacing, where λ is the wavelength of the Gabor signals, performance was poorer than at 4.5 λ (P < 0.01; two-tailed t test) or at 7 λ (P < 0.01). The difference between children and adults in contour-detection performance is greater at larger contour spacings. The main conclusion is that the spatial range of long-range interactions might be limited in young children. The result also indicates that it is not some kind of search deficiency (26) that limits children’s performance, because that should result in an opposite tendency (improving performance with decreasing number of distractors). The third implication is that our effect is probably truly perceptual, because motivational factors (such as perseverance in completing a difficult task) or cognitive mechanisms (such as search strategies) would not be expected to generate different tendencies among children and adults in terms of contour spacing.
Experiment 3: Cue-Specific Learning in the Contour-Integration Task
To test further the involvement of low-level visual mechanisms in the contour-detection task, we looked at the effect of practice and the specificity of learning. Performance in various low-level visual tasks [e.g., hyperacuity (27), visual discrimination (28), pop-out (29), and visual-search tasks (30)] has been shown to improve significantly with practice. Although these tasks involve a variety of visual stimuli, most of them seem to be specific for stimulus parameters (27–29), and some of them seem to be specific even for retinal location (28–29). A high degree of stimulus specificity usually suggests that the plastic neuronal changes of learning took place at early cortical levels in which the basic stimulus dimensions are still separable. We tested whether learning is specific for the stimulus dimensions of orientation and color in the contour-detection task. The lack of transfer of learning across these cues would indicate that the involved mechanisms are early perceptual mechanisms.
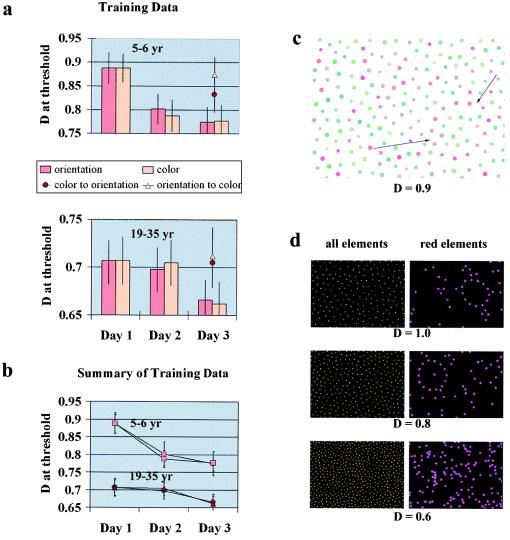
Children (n = 60; 5–6 years) and adults (n = 60; 19–35 years) participated in the learning study with an equal number of observers in the four tested groups. First, we generated a different set of cards with an increased range of D, in which D varied between 1.2 and 0.5. We tested a group of observers (orientation group) with the new set on consecutive days and found significantly improved performance by the third day of practice, as shown in Fig. 3 a and b. The improvement was more evident in children (first- and third-day performances compared: P < 0.01; two-tailed t test) than in adults (P < 0.05). To determine the specificity of this learning effect, we then generated another set of cards in which the path of the contour was defined by color instead of orientation (see Fig. 3 c and d). We calibrated the color cards to match exactly the difficulty levels of the corresponding orientation-defined cards (n = 156, only adults). This procedure provided us with essentially the same task demands for both the orientation- and the color-defined cards. A group of observers (the color group) was tested with the color-defined cards on 3 consecutive days. Learning in the color group was similar to learning in the orientation group both in children (first- and third-day performances compared: P < 0.01) and in adults (P < 0.05). To test whether the improvement transfers from color to orientation, we had a group of observers practice with the color-defined cards for 2 days and tested them with orientation-defined cards on the third day (color-to-orientation group). The last group practiced with the orientation-defined cards for 2 days and was tested with the color cards on the third day (orientation-to-color group). We found that experience with color cards did not significantly improve performance with orientation-defined cards, and vice versa. The transfer was lacking completely in adults in both the color-to-orientation and orientation-to-color groups, as shown by the third-day performances of these groups (Fig. 3a). There was a slight, but not significant, tendency for transfer in children in the color-to-orientation group. First-day orientation and third-day color-to-orientation performances are not significantly different (P = 0.073); third-day orientation and third-day color-to-orientation performances are significantly different (P < 0.05). This tendency might indicate that the contribution of cognitive/motivational factors is present in children to a certain extent; however, the tendency does not explain our data.
Figure 3.
Cue-specific learning in the contour-integration task. (a) Learning curves and transfer of learning results for the groups of 5- to 6-year-old children (Upper) and adults (Lower). Four groups of subjects were tested with the orientation-defined cards on 3 consecutive days (orientation group), with the color-defined cards on 3 consecutive days (color group), with the color cards for 2 days and the orientation cards on the third day (color-to-orientation group), or with the orientation cards for 2 days and the color cards on the third day (orientation-to-color group). In both the orientation and color groups, there was significant learning in children and in adults by the third day of practice. This learning did not transfer significantly between color and orientational cues. Third-day performances in the color-to-orientation and orientation-to-color groups were close to the first-day performances of the other two groups. (b) Summary of the training data in the orientation (circles) and color (squares and triangles) groups. Presenting the results of the two age groups in the same graph reveals that children learn both more and more quickly than adults. (c) An example of the color-defined integration cards. Positions of the elements were taken from the orientation-defined set, and the Gabor signals were replaced by dots to remove orientational information. Information about the contour is now provided by similarity of color. The contour is composed of all red dots, and the background is red and green. The size and luminance of the dots were randomized to ensure that the contour is defined purely by color similarity and not by luminance similarity. (d) The color cards were calibrated to match exactly the difficulty levels of the corresponding orientation-defined cards by varying the density of the red dots in the background. (Left) The positions of elements for three difficulty levels, in which D = 1.0, 0.8, or 0.6 (notice that these positions are the same for the orientation- and color-defined cards). (Right) The positions of the red elements in the color-defined cards.
Because the same set of orientation-defined cards were used in the learning study and because the contour locations were equivalent in the orientation- and color-defined cards, we completed a control experiment to make sure that our subjects are not simply memorizing the locations of the contours in each card. We used one set of orientation-defined cards on 2 consecutive days and tested our subjects with a different set of orientation-defined cards on the third day. The difficulty level of the new set was the same as that of the original set in terms of relative noise density, but the contour shapes and locations were different. We found complete transfer across the two sets; therefore, we concluded that learning in this task is not based simply on memorizing the sequence of contour locations. We also tested the eye specificity of learning by training one eye on 2 consecutive days and the other eye on the third day (n = 10; 5- to 6-year-old children). We found complete interocular transfer, which indicates that learning must have occurred in the cortex at a level in which information from the two eyes is combined and the contribution from subcortical structures is insubstantial.
We conclude that there are similar trends in the adult and child data. Both age groups improve with practice, and they both lack transfer of learning across visual cues. The similar trends suggest that the same mechanisms might be responsible for the performance in both groups. The lack of transfer across cues clearly suggests that the involved mechanisms have access to relatively low-level perceptual representations. Notice that there are no luminance cues on either set of cards. Therefore, integration within specifically tuned mechanisms—such as the orientation- and color-processing “channels”—is required to solve the tasks. The lack of transfer indicates that the dynamic changes during learning must have occurred within these channels.
Discussion
Visual development has been assumed to be completed by the second year in life. Our studies presented here do not support this assumption: integration of contours against a dense noise field shows significant development between 5 and 14 years of age. What determines performance in the contour-detection task? Is the observed handicap in children purely perceptual, or is it caused by high-level cognitive developmental factors, such as less efficient search strategies, or by nonvisual factors, such as less motivation in completing a difficult task? Relying on previous data on the cue specificity of learning in low-level visual tasks (27–29), we estimated the contribution of perceptual mechanisms in the contour-integration performance of 5- to 6-year-old children and adults by employing a learning paradigm. Just as in other low-level visual tasks (27–30), we found that practice led to significant improvement, which was specific for the visual cue defining the task. The cue specificity of learning found in our studies supports the idea that our subjects’ task performance depended on the state of development of their low-level perceptual mechanisms.
What are these perceptual mechanisms? Integration of orientational information across space in the contour-detection task has been related to long-range facilitatory interactions between orientation-tuned spatial channels (15–20). Because of the presence of orientational noise, our task forces the observer to rely on low-level spatial interactions to connect the path of the contour. These interactions might be the facilitatory type mentioned above, but recurrent inhibition might also be appropriate. In either case, cooperative interactions between local processing elements are assumed to be essential in the task. On the other hand, contribution from higher levels of processing cannot be excluded. In a limited-cue configuration—such as in our task—high-level perceptual interpretations function to make sense of the stimulus. It might well be that young children cannot generate and apply these interpretations that easily.
According to our results, the maturational period of cooperative spatial interactions extends well into childhood. We have also found that the performance of children in our task is limited by the spatial range of interactions and not by the level of noise or signal-to-noise ratio per se. This finding indicates, again, that development is specific to a particular visual mechanism and not just a product of some more general factors, such as the capability of operating in noise. Our results show that long-range spatial interactions, although probably present at an early age, are not functioning at the adult level in terms of their spatial range. As shown by our results, the strength of interactions can be extended even with a short training in children with normal vision. By employing a similar contour-detection task, it has been shown that the interactions are not only malleable but also vulnerable: abnormal visual experience early in life may alter their architecture (19, 20), resulting in poor contour-detection performance of amblyopic people with their amblyopic eye (19, 20, 31).
A possible anatomical substrate of the long-range spatial interactions is the plexus of intrinsic horizontal connections of the V1 (21–25). Anatomical data show that the development of horizontal connections in layers 2/3 of V1 in humans extends into childhood (6). Similar, horizontally connected V1 circuits may also integrate color information (32). In addition to these lateral connections, the contour-detection task may be assisted by feedback (modulatory) connections that originate in extrastriate cortex and mediate top-down influences. Interestingly, a delayed postnatal development of feedback connections between V2 and V1 has also been indicated in humans (7). Although the lateral and feedback connections of V1 are probably essential in completing our contour-detection task, it has also been shown to involve neural operations extracting “global” shape (15). Therefore, the participation of “intermediate-level” cortical areas concerned with form vision, such as V4 (33), is also expected.
What is the functional relevance of our findings? The long-range connections of the visual cortex are assumed to mediate contextual effects in perception (15, 17, 22, 25, 32, 34). With immature connections, perception should be more local and less affected by context. There is, indeed, indication that perceptual skills mediating contextual effects in pictorial illusions are underdeveloped in young children (35, 36). Further behavioral evidence indicates that these contextual effects might be largely mediated by the occipitotemporal (or ventral) visual stream (37–40). There is also evidence that the functional development of the occipitotemporal pathway is slower than that of the occipitoparietal (or dorsal) pathway in macaque monkeys (41, 42). However, human neuroanatomical evidence remains to be found to support the possibility that the dorsal visual stream matures early and that the ventral visual stream—related to more refined perceptual categorization, object recognition, and contextual effects (43–45)—matures relatively more slowly.
Acknowledgments
We thank Shital Shah, Michelle May, and Zsuzsanna Káldy for helping us with data collection, and Zoltán Vidnyánszky for his helpful comments on the manuscript. This work was supported by the J. S. McDonnell Foundation Grant 9560 and by Országos Tudományos Kutatási Alap (Hungary) Grant 016959.
Abbreviation
- Vn
visual cortex n
References
- 1.Nelson C A, Horowitz F D. In: Handbook of Infant Perception. Salapatek P, Cohen L, editors. Vol. 2. New York: Academic; 1987. pp. 123–153. [Google Scholar]
- 2.Kellman P, Spelke E. Cognit Psychol. 1983;15:483–524. doi: 10.1016/0010-0285(83)90017-8. [DOI] [PubMed] [Google Scholar]
- 3.Bornstein M H, Kessen W, Weiskopf S. Science. 1976;191:201–202. doi: 10.1126/science.1246610. [DOI] [PubMed] [Google Scholar]
- 4.Braddick O J, Atkinson B, Julesz B, Kropfl W, Bodis-Wollner I, Raab E. Nature (London) 1980;288:363–365. doi: 10.1038/288363a0. [DOI] [PubMed] [Google Scholar]
- 5.Dobson V, Teller D Y. Vision Res. 1978;18:1469–1483. doi: 10.1016/0042-6989(78)90001-9. [DOI] [PubMed] [Google Scholar]
- 6.Burkhalter A, Bernardo K L, Charles V. J Neurosci. 1993;13:1916–1931. doi: 10.1523/JNEUROSCI.13-05-01916.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burkhalter A. Cereb Cortex. 1993;3:475–487. doi: 10.1093/cercor/3.5.476. [DOI] [PubMed] [Google Scholar]
- 8.Shankle W R, Landing B H, Rafii M S, Schiano A, Chen J M, Hara J. J Theor Biol. 1998;191:115–140. doi: 10.1006/jtbi.1997.0570. [DOI] [PubMed] [Google Scholar]
- 9.Shankle W R, Romney A K, Landing B H, Hara J. Proc Natl Acad Sci USA. 1998;95:4023–4028. doi: 10.1073/pnas.95.7.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Atkinson J, Braddick O. Behav Brain Res. 1993;49:123–131. doi: 10.1016/s0166-4328(05)80202-5. [DOI] [PubMed] [Google Scholar]
- 11.Sireteanu R, Rieth C. Behav Brain Res. 1993;49:133–139. doi: 10.1016/s0166-4328(05)80203-7. [DOI] [PubMed] [Google Scholar]
- 12.Hollants-Gilhuijs M A, Ruijter J M, Spekreijse H. Vision Res. 1998;38:651–657. doi: 10.1016/s0042-6989(97)00202-2. [DOI] [PubMed] [Google Scholar]
- 13.Hollants-Gilhuijs M A, Ruijter J M, Spekreijse H. Vision Res. 1998;38:645–649. doi: 10.1016/s0042-6989(97)00203-4. [DOI] [PubMed] [Google Scholar]
- 14.Gollin E S. Percept Mot Skills. 1960;11:289–298. [Google Scholar]
- 15.Kovács I, Julesz B. Proc Natl Acad Sci USA. 1993;90:7495–7497. doi: 10.1073/pnas.90.16.7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Field D J, Hayes A, Hess R F. Vision Res. 1993;33:173–193. doi: 10.1016/0042-6989(93)90156-q. [DOI] [PubMed] [Google Scholar]
- 17.Kovács I. Behav Brain Res. 1996;82:1–11. doi: 10.1016/s0166-4328(97)81103-5. [DOI] [PubMed] [Google Scholar]
- 18.Dakin S C, Hess R F. J Opt Soc Am. 1998;15:1486–1499. doi: 10.1364/josaa.15.001486. [DOI] [PubMed] [Google Scholar]
- 19.Kovács I, Polat U, Norcia A M. Invest Ophthalmol Visual Sci Suppl. 1996;37:670. (abstr.). [Google Scholar]
- 20.Pennefather, P. M., Chandna, A., Kovács, I., Polat, U. & Norcia, A. M. (1999) Spat. Vis., in press. [DOI] [PubMed]
- 21.Rockland K S, Lund J S. Science. 1982;215:1532–1534. doi: 10.1126/science.7063863. [DOI] [PubMed] [Google Scholar]
- 22.Gilbert C D, Wiesel T N. J Neurosci. 1983;3:1116–1133. doi: 10.1523/JNEUROSCI.03-05-01116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitchison G J, Crick F. Proc Natl Acad Sci USA. 1982;79:3661–3665. doi: 10.1073/pnas.79.11.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nelson J I, Frost B J. Exp Brain Res. 1985;61:54–61. doi: 10.1007/BF00235620. [DOI] [PubMed] [Google Scholar]
- 25.Gilbert C D. Physiol Rev. 1998;78:467–485. doi: 10.1152/physrev.1998.78.2.467. [DOI] [PubMed] [Google Scholar]
- 26.Sireteanu R, Rettenbach R. Klin Monatsbl Augenheilkd. 1996;208:3–10. doi: 10.1055/s-2008-1035160. [DOI] [PubMed] [Google Scholar]
- 27.Poggio T, Fahle M, Edelman S. Science. 1992;256:1018–1021. doi: 10.1126/science.1589770. [DOI] [PubMed] [Google Scholar]
- 28.Fiorentini A, Berardi N. Nature (London) 1980;287:43–44. doi: 10.1038/287043a0. [DOI] [PubMed] [Google Scholar]
- 29.Karni A, Sagi D. Nature (London) 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 30.Sireteanu R, Rettenbach R. Vision Res. 1995;35:2037–2043. doi: 10.1016/0042-6989(94)00295-w. [DOI] [PubMed] [Google Scholar]
- 31.Hess R F, McIlhagga W H, Field D J. Vision Res. 1997;37:3145–3161. doi: 10.1016/s0042-6989(96)00281-7. [DOI] [PubMed] [Google Scholar]
- 32.Ts’o D Y, Gilbert C D. J Neurosci. 1988;8:1712–1727. doi: 10.1523/JNEUROSCI.08-05-01712.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilson H R, Wilkinson F. Vision Res. 1998;38:2933–2947. doi: 10.1016/s0042-6989(98)00109-6. [DOI] [PubMed] [Google Scholar]
- 34.Kovács I, Julesz B. Nature (London) 1994;370:644–646. doi: 10.1038/370644a0. [DOI] [PubMed] [Google Scholar]
- 35.Weintraub D J. J Exp Psychol Hum Percept Perform. 1979;5:353–364. doi: 10.1037//0096-1523.5.2.353. [DOI] [PubMed] [Google Scholar]
- 36.Zanutti L. Percept Mot Skills. 1996;82:15–18. doi: 10.2466/pms.1996.82.1.15. [DOI] [PubMed] [Google Scholar]
- 37.Marotta J J, De Souza J F X, Haffenden A M, Goodale M A. Neuropsychologia. 1998;36:491–497. doi: 10.1016/s0028-3932(97)00154-1. [DOI] [PubMed] [Google Scholar]
- 38.Haffenden A, Goodale M A. J Cogn Neuroscience. 1998;10:122–136. doi: 10.1162/089892998563824. [DOI] [PubMed] [Google Scholar]
- 39.Goodale M A, Haffenden A. Neurosci Behav Rev. 1998;22:161–172. doi: 10.1016/s0149-7634(97)00007-9. [DOI] [PubMed] [Google Scholar]
- 40.Goodale M A, Humphrey G K. Cognition. 1998;67:181–207. doi: 10.1016/s0010-0277(98)00017-1. [DOI] [PubMed] [Google Scholar]
- 41.Bachevalier J, Hagger C, Mishkin M. In: Brain Work and Mental Activity, Alfred Benzon Symposium. Lassen N A, Ingvar D H, Raichle M E, Friberg L, editors. Vol. 31. Copenhagen: Munksgaard; 1991. pp. 231–240. [Google Scholar]
- 42.Distler C, Bachevalier J, Kennedy C, Mishkin M, Ungerleider L G. Cereb Cortex. 1996;6:184–195. doi: 10.1093/cercor/6.2.184. [DOI] [PubMed] [Google Scholar]
- 43.Ungerleider L G, Mishkin M. In: Analysis of Visual Behavior. Ingle D J, Goodale M A, Mansfield R J W, editors. Cambridge, MA: MIT Press; 1982. pp. 549–586. [Google Scholar]
- 44.Goodale M A, Milner A D. Trends Neurosci. 1992;15:20–25. doi: 10.1016/0166-2236(92)90344-8. [DOI] [PubMed] [Google Scholar]
- 45.Milner A D, Goodale M A. The Visual Brain in Action. Oxford: Oxford Univ. Press; 1995. [Google Scholar]