Abstract
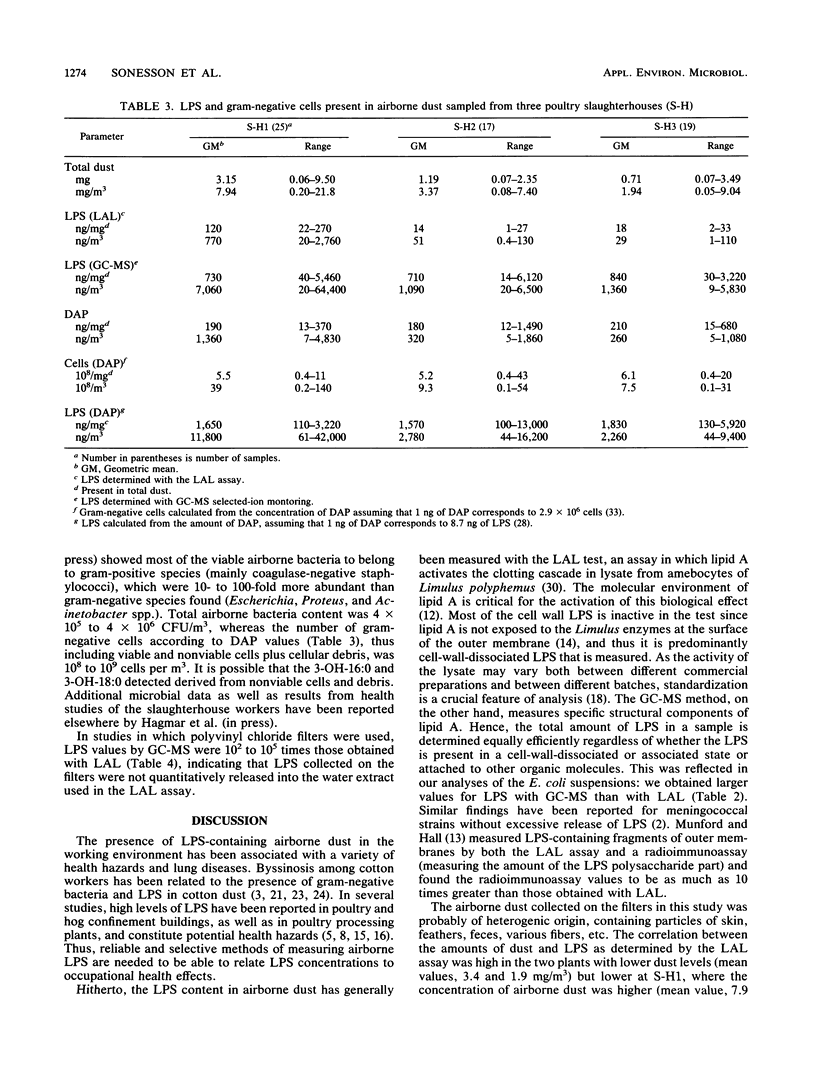
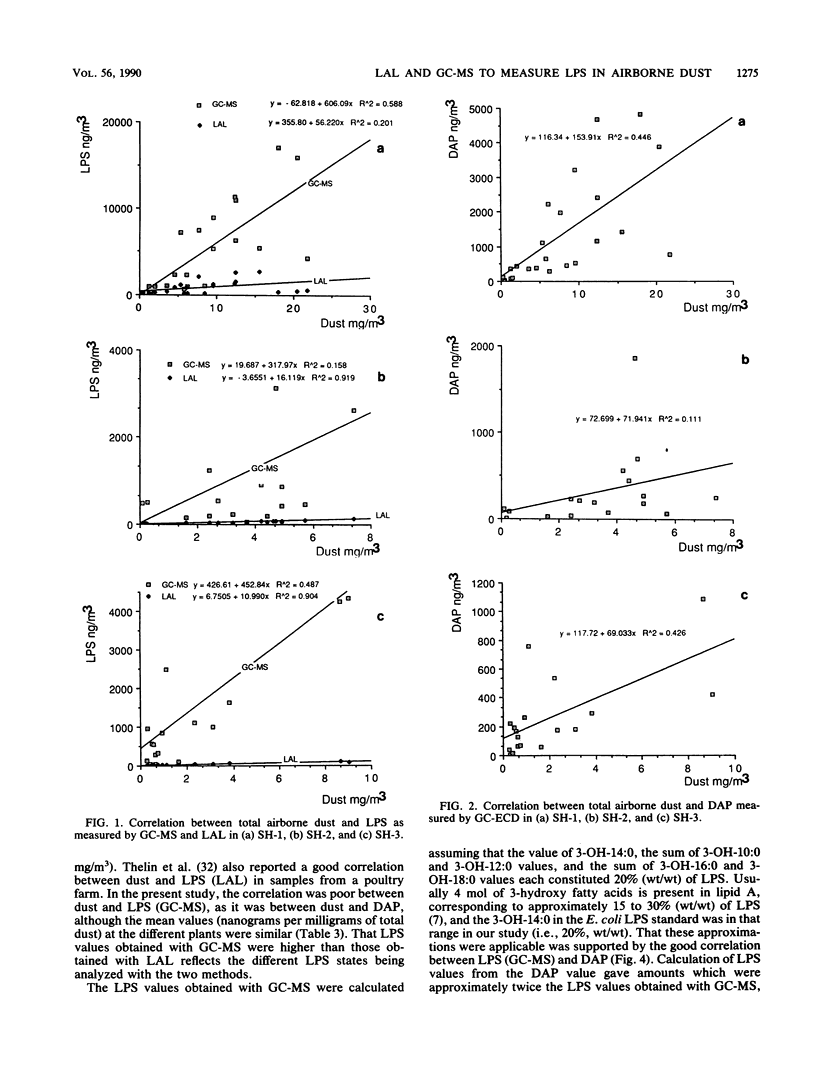
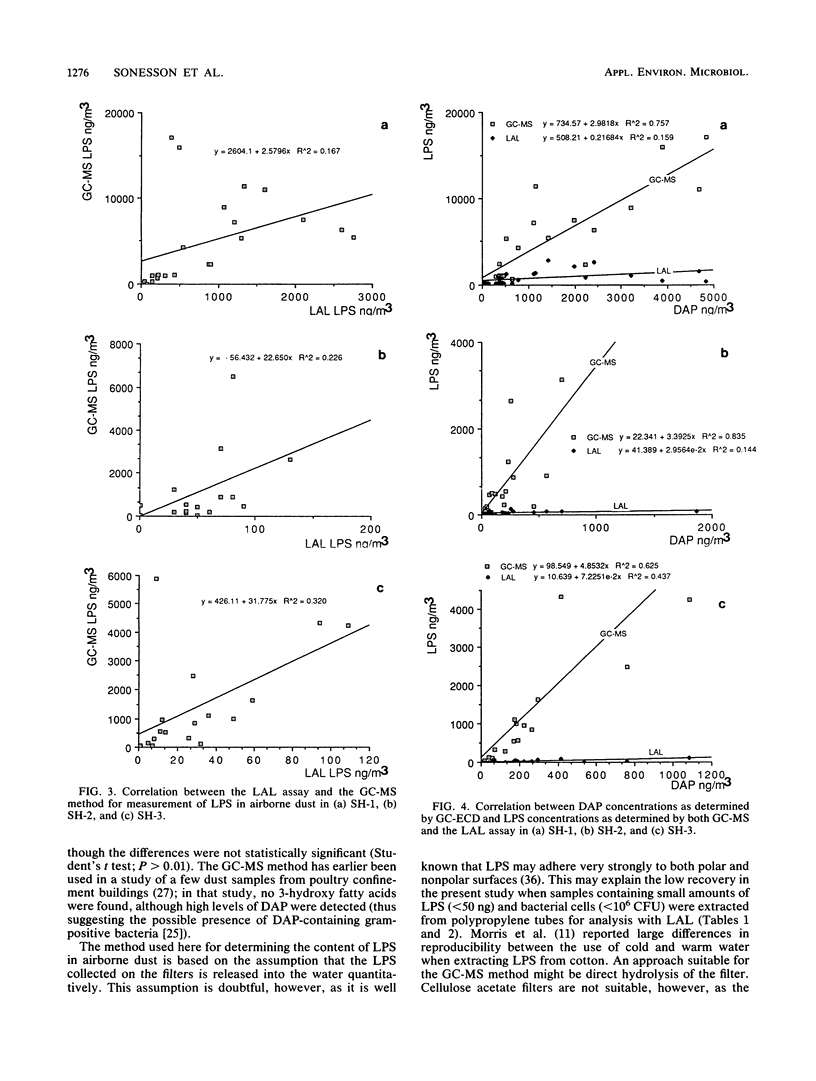
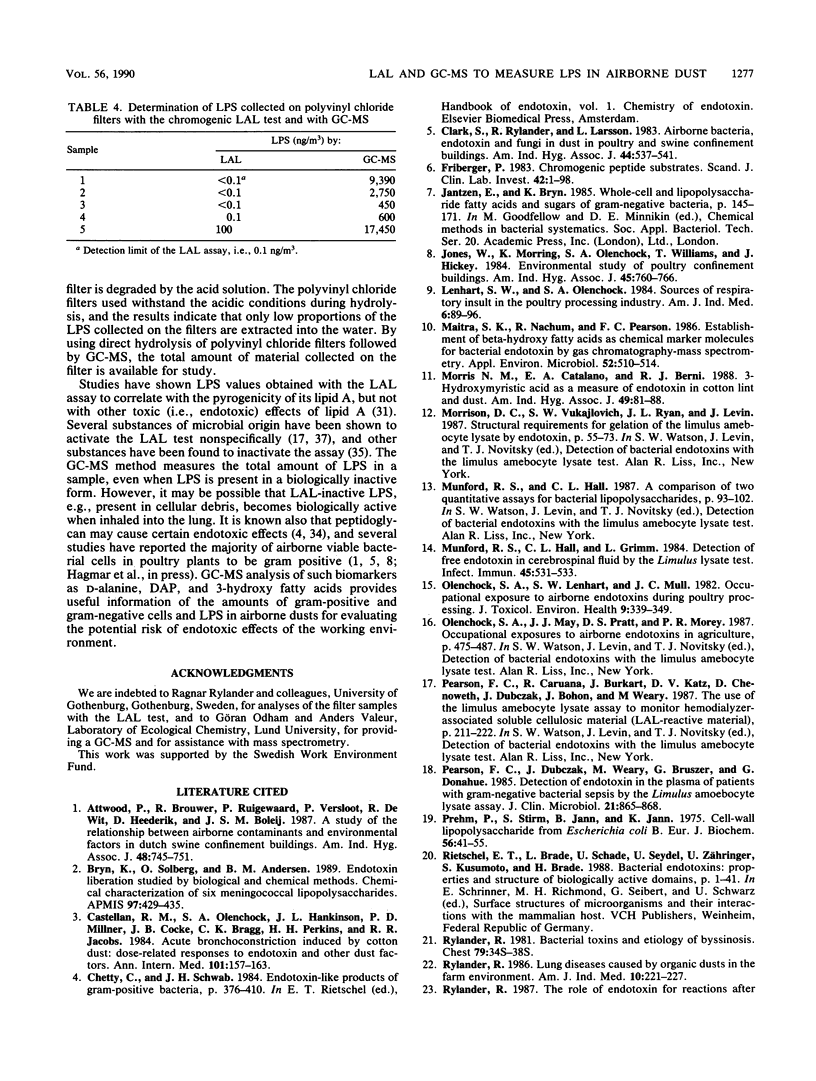
The lipopolysaccharide (endotoxin) content in airborne dust samples from three different poultry slaughterhouses was determined with both the chromogenic Limulus amebocyte lysate assay and gas chromatography-mass spectrometry analysis of lipopolysaccharide-derived 3-hydroxy fatty acids. Gram-negative cell walls were also measured by using two-dimensional gas chromatography/electron-capture analysis of diaminopimelic acid originating from the peptidoglycan. The correlation between the results of the Limulus assay and those of gas chromatography-mass spectrometry for determination of the lipopolysaccharide content in the dust samples was poor, whereas a good correlation was obtained between lipopolysaccharide and diaminopimelic acid concentrations with the gas chromatographic methods. The results suggest that it is predominantly cell-wall-dissociated lipopolysaccharides that are measured with the Limulus assay, whereas the gas chromatographic methods allow determination of total concentrations of lipopolysaccharide, including Limulus-inactive lipopolysaccharide, gram-negative cells, and cellular debris.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwood P., Brouwer R., Ruigewaard P., Versloot P., de Wit R., Heederik D., Boleij J. S. A study of the relationship between airborne contaminants and environmental factors in Dutch swine confinement buildings. Am Ind Hyg Assoc J. 1987 Aug;48(8):745–751. doi: 10.1080/15298668791385507. [DOI] [PubMed] [Google Scholar]
- Bryn K., Solberg O., Andersen B. M. Endotoxin liberation studied by biological and chemical methods. Chemical characterization of six meningococcal lipopolysaccharides. APMIS. 1989 May;97(5):429–435. doi: 10.1111/j.1699-0463.1989.tb00811.x. [DOI] [PubMed] [Google Scholar]
- Castellan R. M., Olenchock S. A., Hankinson J. L., Millner P. D., Cocke J. B., Bragg C. K., Perkins H. H., Jr, Jacobs R. R. Acute bronchoconstriction induced by cotton dust: dose-related responses to endotoxin and other dust factors. Ann Intern Med. 1984 Aug;101(2):157–163. doi: 10.7326/0003-4819-101-2-157. [DOI] [PubMed] [Google Scholar]
- Clark S., Rylander R., Larsson L. Airborne bacteria, endotoxin and fungi in dust in poultry and swine confinement buildings. Am Ind Hyg Assoc J. 1983 Jul;44(7):537–541. doi: 10.1080/15298668391405265. [DOI] [PubMed] [Google Scholar]
- Jones W., Morring K., Olenchock S. A., Williams T., Hickey J. Environmental study of poultry confinement buildings. Am Ind Hyg Assoc J. 1984 Nov;45(11):760–766. [PubMed] [Google Scholar]
- Lenhart S. W., Olenchock S. A. Sources of respiratory insult in the poultry processing industry. Am J Ind Med. 1984;6(2):89–96. doi: 10.1002/ajim.4700060203. [DOI] [PubMed] [Google Scholar]
- Maitra S. K., Nachum R., Pearson F. C. Establishment of beta-hydroxy fatty acids as chemical marker molecules for bacterial endotoxin by gas chromatography-mass spectrometry. Appl Environ Microbiol. 1986 Sep;52(3):510–514. doi: 10.1128/aem.52.3.510-514.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris N. M., Catalano E. A., Berni R. J. 3-Hydroxymyristic acid as a measure of endotoxin in cotton lint and dust. Am Ind Hyg Assoc J. 1988 Feb;49(2):81–88. doi: 10.1080/15298668891379431. [DOI] [PubMed] [Google Scholar]
- Morrison D. C., Vukajlovich S. W., Ryan J. L., Levin J. Structural requirements for gelation of the Limulus amebocyte lysate by endotoxin. Prog Clin Biol Res. 1987;231:55–73. [PubMed] [Google Scholar]
- Munford R. S., Hall C. L. A comparison of two quantitative assays for bacterial lipopolysaccharides. Prog Clin Biol Res. 1987;231:93–102. [PubMed] [Google Scholar]
- Munford R. S., Hall C. L., Grimm L. Detection of free endotoxin in cerebrospinal fluid by the Limulus lysate test. Infect Immun. 1984 Aug;45(2):531–533. doi: 10.1128/iai.45.2.531-533.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olenchock S. A., Lenhart S. W., Mull J. C. Occupational exposure to airborne endotoxins during poultry processing. J Toxicol Environ Health. 1982 Feb;9(2):339–349. doi: 10.1080/15287398209530166. [DOI] [PubMed] [Google Scholar]
- Olenchock S. A., May J. J., Pratt D. S., Morey P. R. Occupational exposures to airborne endotoxins in agriculture. Prog Clin Biol Res. 1987;231:475–487. [PubMed] [Google Scholar]
- Pearson F. C., Caruana R., Burkart J., Katz D. V., Chenoweth D., Dubczak J., Bohon J., Weary M. The use of the Limulus amebocyte lysate assay to monitor hemodialyzer-associated soluble cellulosic material (LAL-reactive material). Prog Clin Biol Res. 1987;231:211–222. [PubMed] [Google Scholar]
- Pearson F. C., Dubczak J., Weary M., Bruszer G., Donohue G. Detection of endotoxin in the plasma of patients with gram-negative bacterial sepsis by the Limulus amoebocyte lysate assay. J Clin Microbiol. 1985 Jun;21(6):865–868. doi: 10.1128/jcm.21.6.865-868.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prehm P., Stirm S., Jann B., Jann K. Cell-wall lipopolysaccharide from Escherichia coli B. Eur J Biochem. 1975 Aug 1;56(1):41–55. doi: 10.1111/j.1432-1033.1975.tb02205.x. [DOI] [PubMed] [Google Scholar]
- Rylander R. Bacterial toxins and etiology of byssinosis. Chest. 1981 Apr;79(4 Suppl):34S–38S. doi: 10.1378/chest.79.4_supplement.34s. [DOI] [PubMed] [Google Scholar]
- Rylander R., Haglind P., Lundholm M. Endotoxin in cotton dust and respiratory function decrement among cotton workers in an experimental cardroom. Am Rev Respir Dis. 1985 Feb;131(2):209–213. doi: 10.1164/arrd.1985.131.2.209. [DOI] [PubMed] [Google Scholar]
- Rylander R. Lung diseases caused by organic dusts in the farm environment. Am J Ind Med. 1986;10(3):221–227. doi: 10.1002/ajim.4700100306. [DOI] [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonesson A., Bryn K., Jantzen E., Larsson L. Gas chromatographic determination of (phosphorylated) 2-keto-3-deoxyoctonic acid, heptoses and glucosamine in bacterial lipopolysaccharides after treatment with hydrofluoric acid, methanolysis and trifluoroacetylation. J Chromatogr. 1989 Jan 27;487(1):1–7. doi: 10.1016/s0378-4347(00)83001-7. [DOI] [PubMed] [Google Scholar]
- Sonesson A., Larsson L., Fox A., Westerdahl G., Odham G. Determination of environmental levels of peptidoglycan and lipopolysaccharide using gas chromatography with negative-ion chemical-ionization mass spectrometry utilizing bacterial amino acids and hydroxy fatty acids as biomarkers. J Chromatogr. 1988 Sep 23;431(1):1–15. doi: 10.1016/s0378-4347(00)83064-9. [DOI] [PubMed] [Google Scholar]
- Sonesson A., Larsson L., Jimenez J. Two-dimensional gas chromatography with electron-capture detection used in the determination of specific peptidoglycan and lipopolysaccharide constituents of gram-negative bacteria in infected human urine. J Chromatogr. 1989 May 5;490(1):71–79. doi: 10.1016/s0378-4347(00)82762-0. [DOI] [PubMed] [Google Scholar]
- Sonesson A., Larsson L., Westerdahl G., Odham G. Determination of endotoxins by gas chromatography: evaluation of electron-capture and negative-ion chemical-ionization mass spectrometric detection of halogenated derivatives of beta-hydroxymyristic acid. J Chromatogr. 1987 Jun 5;417(1):11–25. doi: 10.1016/0378-4347(87)80087-7. [DOI] [PubMed] [Google Scholar]
- Takada H., Kotani S., Tanaka S., Ogawa T., Takahashi I., Tsujimoto M., Komuro T., Shiba T., Kusumoto S., Kusunose N. Structural requirements of lipid A species in activation of clotting enzymes from the horseshoe crab, and the human complement cascade. Eur J Biochem. 1988 Aug 15;175(3):573–580. doi: 10.1111/j.1432-1033.1988.tb14230.x. [DOI] [PubMed] [Google Scholar]
- Takayama K., Qureshi N., Raetz C. R., Ribi E., Peterson J., Cantrell J. L., Pearson F. C., Wiggins J., Johnson A. G. Influence of fine structure of lipid A on Limulus amebocyte lysate clotting and toxic activities. Infect Immun. 1984 Aug;45(2):350–355. doi: 10.1128/iai.45.2.350-355.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin A., Tegler O., Rylander R. Lung reactions during poultry handling related to dust and bacterial endotoxin levels. Eur J Respir Dis. 1984 May;65(4):266–271. [PubMed] [Google Scholar]
- Weiss A. R. A new ultrafiltration unit for the removal of substances interfering with the LAL-endotoxin-test. Prog Clin Biol Res. 1987;231:235–249. [PubMed] [Google Scholar]
- Wicken A. J., Knox K. W. Bacterial cell surface amphiphiles. Biochim Biophys Acta. 1980 May 27;604(1):1–26. doi: 10.1016/0005-2736(80)90583-0. [DOI] [PubMed] [Google Scholar]


