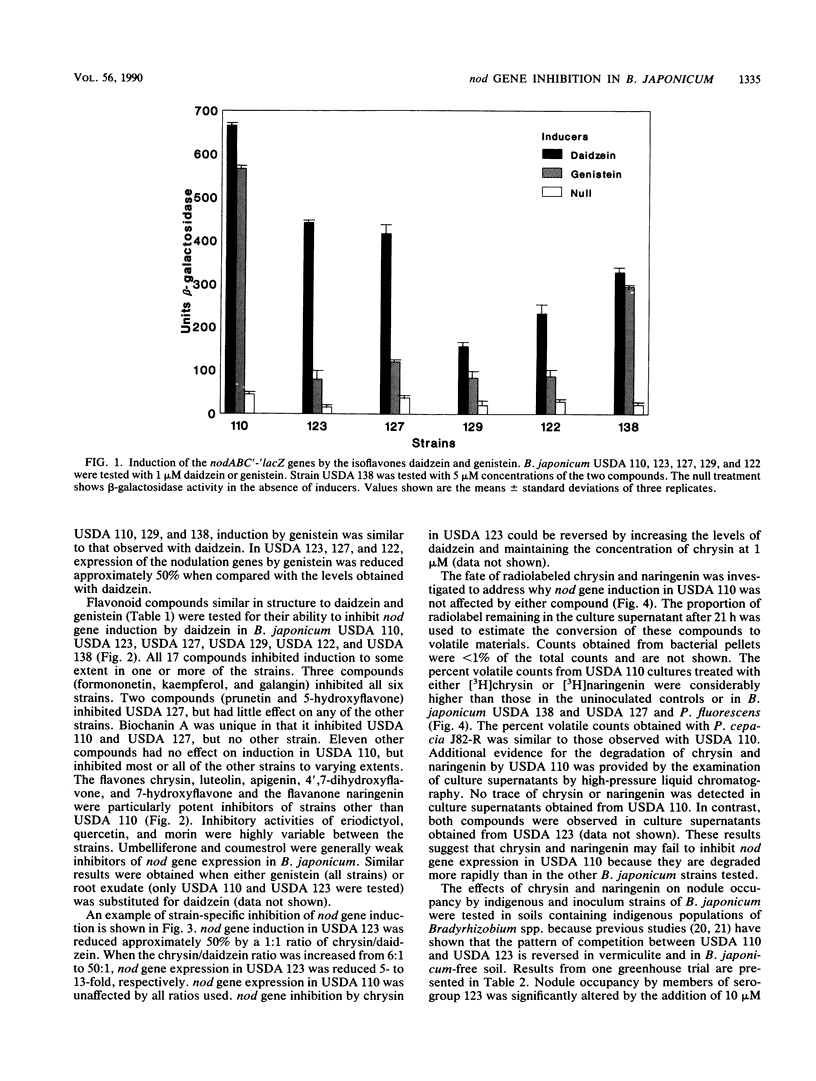
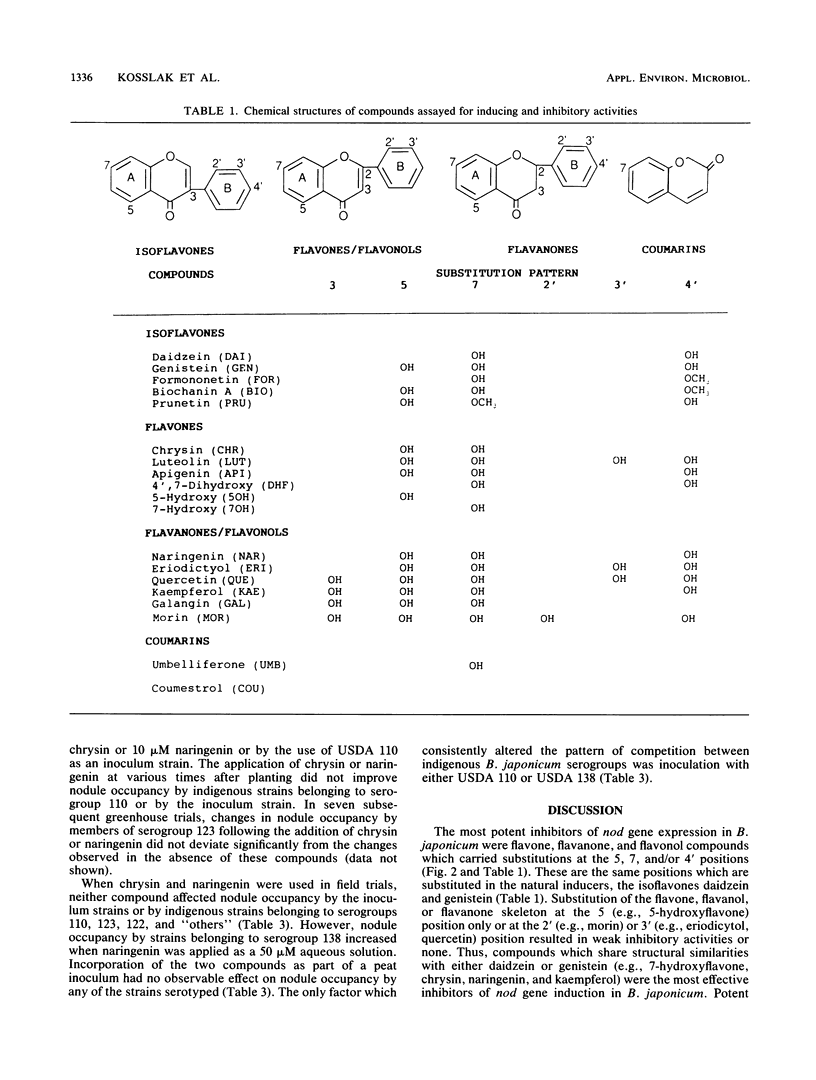
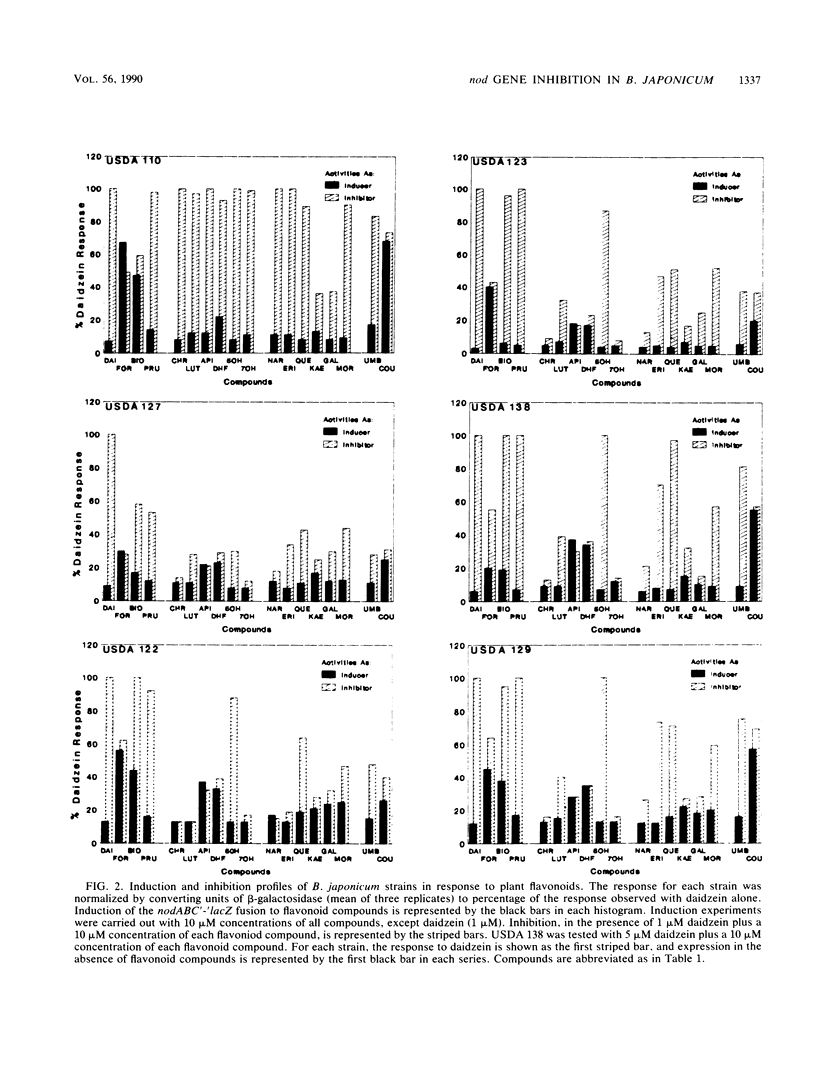
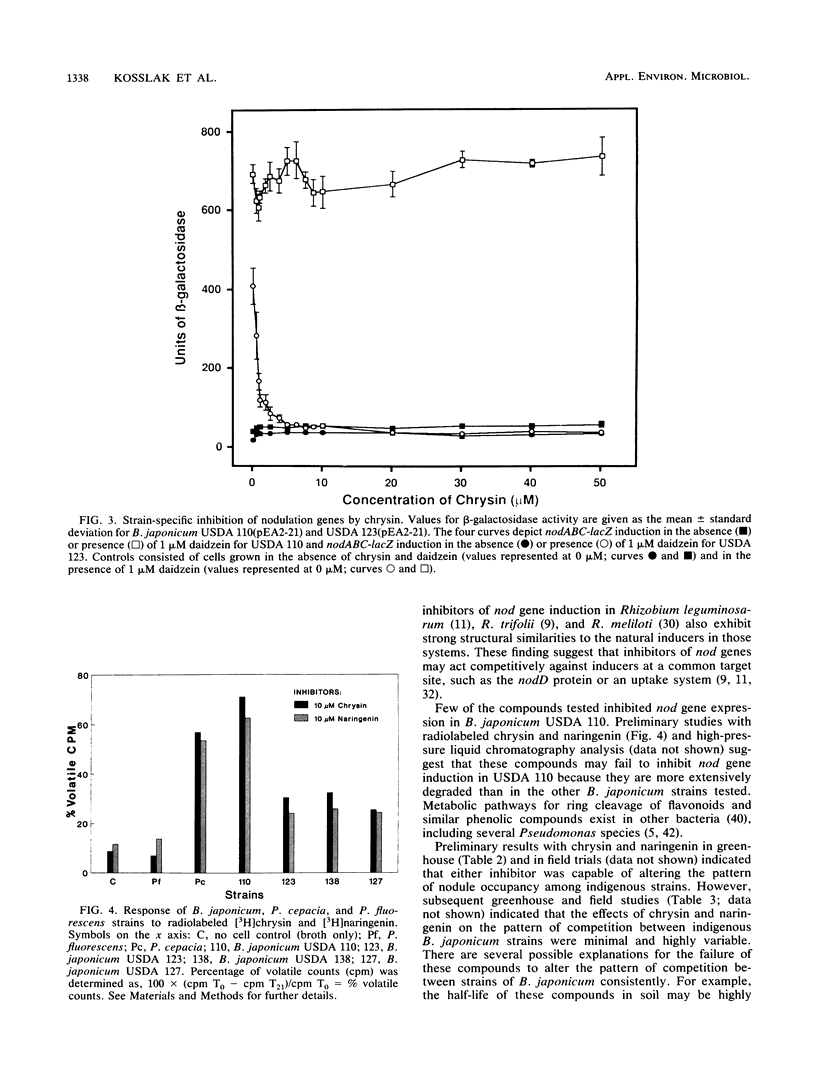
Abstract
A broad-host-range plasmid, pEA2-21, containing a Bradyrhizobium japonicum nodABC'-'lacZ translational fusion was used to identify strain-specific inhibitors of the genes required for soybean nodulation, the common nod genes. The responses of type strains of B. japonicum serogroups USDA 110, USDA 123, USDA 127, USDA 129, USDA 122, and USDA 138 to nod gene inhibitors were compared. Few compounds inhibited nod gene expression in B. japonicum USDA 110. In contrast, nod gene expression in strains belonging to several other serogroups was inhibited by most of the flavonoids tested. However, the application of two of these strain-specific compounds, chrysin and naringenin, had little effect on the pattern of competition between indigenous and inoculum strains of B. japonicum in greenhouse and field trials. Preliminary studies with radiolabeled chrysin and naringenin suggest that the different responses to nod gene inhibitors may be partly due to the degree to which plant flavonoids can be metabolized by each strain.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banfalvi Z., Nieuwkoop A., Schell M., Besl L., Stacey G. Regulation of nod gene expression in Bradyrhizobium japonicum. Mol Gen Genet. 1988 Nov;214(3):420–424. doi: 10.1007/BF00330475. [DOI] [PubMed] [Google Scholar]
- Burlage R. S., Hooper S. W., Sayler G. S. The TOL (pWW0) catabolic plasmid. Appl Environ Microbiol. 1989 Jun;55(6):1323–1328. doi: 10.1128/aem.55.6.1323-1328.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cregan P. B., Keyser H. H. Influence of Glycine spp. on Competitiveness of Bradyrhizobium japonicum and Rhizobium fredii. Appl Environ Microbiol. 1988 Mar;54(3):803–808. doi: 10.1128/aem.54.3.803-808.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic M. A., Redmond J. W., Batley M., Rolfe B. G. Clovers secrete specific phenolic compounds which either stimulate or repress nod gene expression in Rhizobium trifolii. EMBO J. 1987 May;6(5):1173–1179. doi: 10.1002/j.1460-2075.1987.tb02351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dower W. J., Miller J. F., Ragsdale C. W. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 1988 Jul 11;16(13):6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath B., Bachem C. W., Schell J., Kondorosi A. Host-specific regulation of nodulation genes in Rhizobium is mediated by a plant-signal, interacting with the nodD gene product. EMBO J. 1987 Apr;6(4):841–848. doi: 10.1002/j.1460-2075.1987.tb04829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamicker B. J., Brill W. J. Identification of Bradyrhizobium japonicum Nodule Isolates from Wisconsin Soybean Farms. Appl Environ Microbiol. 1986 Mar;51(3):487–492. doi: 10.1128/aem.51.3.487-492.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyser H. H., Cregan P. B. Nodulation and Competition for Nodulation of Selected Soybean Genotypes among Bradyrhizobium japonicum Serogroup 123 Isolates. Appl Environ Microbiol. 1987 Nov;53(11):2631–2635. doi: 10.1128/aem.53.11.2631-2635.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishinevsky B., Bar-Joseph M. Rhizobium strain identification in Arachis hypogaea nodules by enzyme-linked immunosorbent assay (ELISA). Can J Microbiol. 1978 Dec;24(12):1537–1543. doi: 10.1139/m78-245. [DOI] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B., Dowdle S., Sadowsky M. J. Competition of Rhizobium japonicum Strains in Early Stages of Soybean Nodulation. Appl Environ Microbiol. 1983 Oct;46(4):870–873. doi: 10.1128/aem.46.4.870-873.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Influence of Environmental Factors on Interstrain Competition in Rhizobium japonicum. Appl Environ Microbiol. 1985 May;49(5):1128–1133. doi: 10.1128/aem.49.5.1128-1133.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bohlool B. B. Suppression of nodule development of one side of a split-root system of soybeans caused by prior inoculation of the other side. Plant Physiol. 1984 May;75(1):125–130. doi: 10.1104/pp.75.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosslak R. M., Bookland R., Barkei J., Paaren H. E., Appelbaum E. R. Induction of Bradyrhizobium japonicum common nod genes by isoflavones isolated from Glycine max. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7428–7432. doi: 10.1073/pnas.84.21.7428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long S. R. Rhizobium-legume nodulation: life together in the underground. Cell. 1989 Jan 27;56(2):203–214. doi: 10.1016/0092-8674(89)90893-3. [DOI] [PubMed] [Google Scholar]
- McIver J., Djordjevic M. A., Weinman J. J., Bender G. L., Rolfe B. G. Extension of host range of Rhizobium leguminosarum bv. trifolii caused by point mutations in nodD that result in alterations in regulatory function and recognition of inducer molecules. Mol Plant Microbe Interact. 1989 May-Jun;2(3):97–106. doi: 10.1094/mpmi-2-097. [DOI] [PubMed] [Google Scholar]
- McLoughlin T. J., Merlo A. O., Satola S. W., Johansen E. Isolation of competition-defective mutants of Rhizobium fredii. J Bacteriol. 1987 Jan;169(1):410–413. doi: 10.1128/jb.169.1.410-413.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moawad H. A., Ellis W. R., Schmidt E. L. Rhizosphere Response as a Factor in Competition Among Three Serogroups of Indigenous Rhizobium japonicum for Nodulation of Field-Grown Soybeans. Appl Environ Microbiol. 1984 Apr;47(4):607–612. doi: 10.1128/aem.47.4.607-612.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan J. T., Long S. R. Induction of Rhizobium meliloti nodC expression by plant exudate requires nodD. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6609–6613. doi: 10.1073/pnas.82.19.6609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters N. K., Long S. R. Alfalfa Root Exudates and Compounds which Promote or Inhibit Induction of Rhizobium meliloti Nodulation Genes. Plant Physiol. 1988 Oct;88(2):396–400. doi: 10.1104/pp.88.2.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky M. J., Tully R. E., Cregan P. B., Keyser H. H. Genetic Diversity in Bradyrhizobium japonicum Serogroup 123 and Its Relation to Genotype-Specific Nodulation of Soybean. Appl Environ Microbiol. 1987 Nov;53(11):2624–2630. doi: 10.1128/aem.53.11.2624-2630.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaman H. R., Spaink H. P., Okker R. J., Lugtenberg B. J. Subcellular localization of the nodD gene product in Rhizobium leguminosarum. J Bacteriol. 1989 Sep;171(9):4686–4693. doi: 10.1128/jb.171.9.4686-4693.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt E. L., Zidwick M. J., Abebe H. M. Bradyrhizobium japonicum Serocluster 123 and Diversity among Member Isolates. Appl Environ Microbiol. 1986 Jun;51(6):1212–1215. doi: 10.1128/aem.51.6.1212-1215.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singleton P. W., Tavares J. W. Inoculation response of legumes in relation to the number and effectiveness of indigenous Rhizobium populations. Appl Environ Microbiol. 1986 May;51(5):1013–1018. doi: 10.1128/aem.51.5.1013-1018.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triplett E. W. Isolation of genes involved in nodulation competitiveness from Rhizobium leguminosarum bv. trifolii T24. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3810–3814. doi: 10.1073/pnas.85.11.3810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter J., Moore L. H., Dowell V. R., Jr, Bokkenheuser V. D. C-ring cleavage of flavonoids by human intestinal bacteria. Appl Environ Microbiol. 1989 May;55(5):1203–1208. doi: 10.1128/aem.55.5.1203-1208.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaat S. A., Wijffelman C. A., Mulders I. H., van Brussel A. A., Lugtenberg B. J. Root Exudates of Various Host Plants of Rhizobium leguminosarum Contain Different Sets of Inducers of Rhizobium Nodulation Genes. Plant Physiol. 1988 Apr;86(4):1298–1303. doi: 10.1104/pp.86.4.1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylstra G. J., McCombie W. R., Gibson D. T., Finette B. A. Toluene degradation by Pseudomonas putida F1: genetic organization of the tod operon. Appl Environ Microbiol. 1988 Jun;54(6):1498–1503. doi: 10.1128/aem.54.6.1498-1503.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]



