Abstract
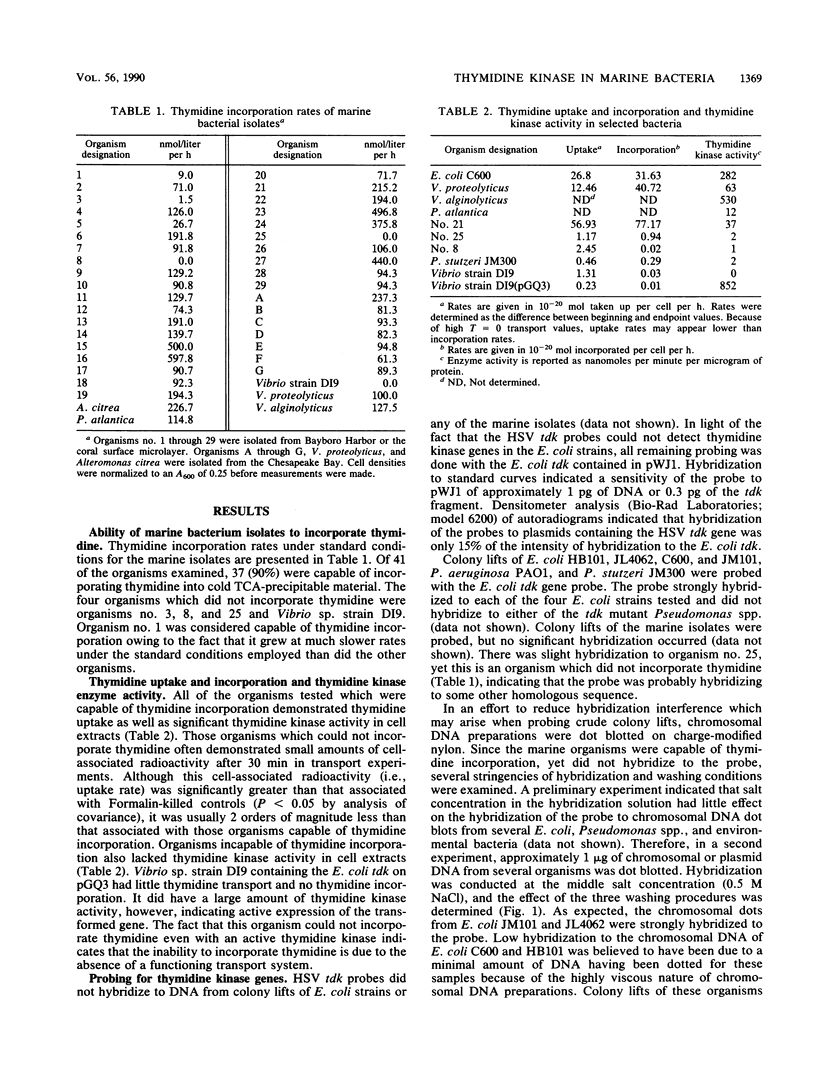
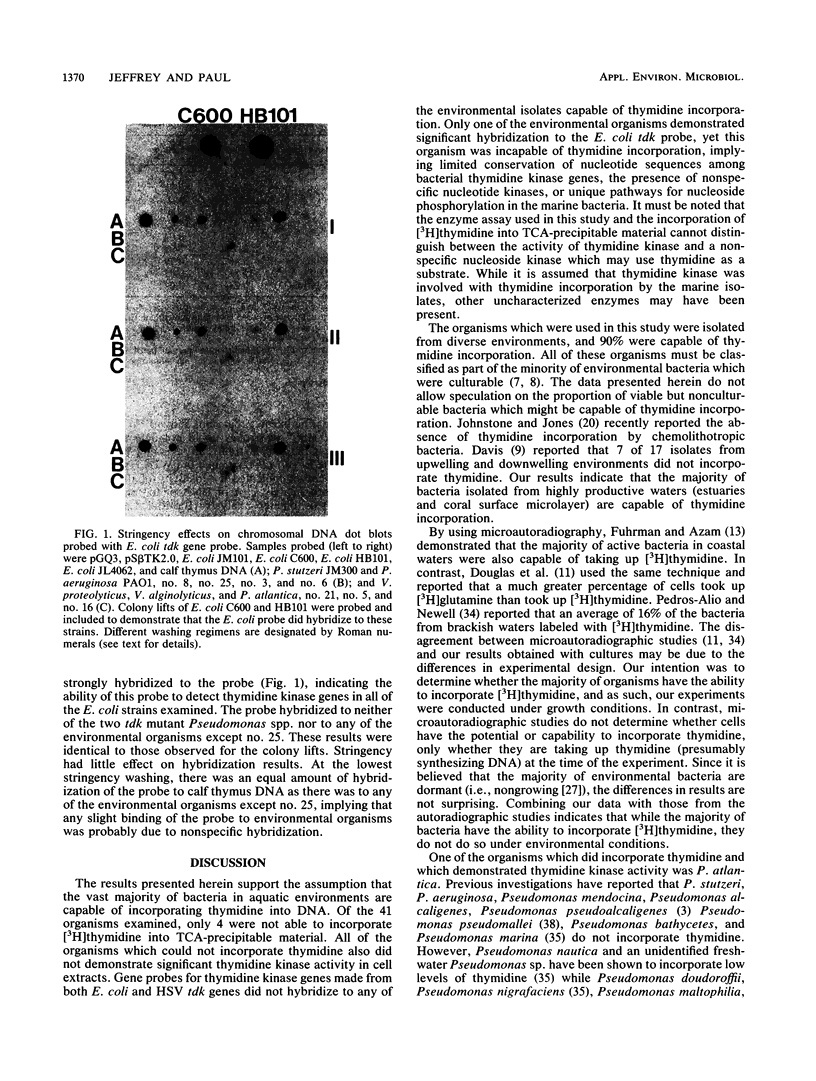
One assumption made in bacterial production estimates from [3H]thymidine incorporation is that all heterotrophic bacteria can incorporate exogenous thymidine into DNA. Heterotrophic marine bacterium isolates from Tampa Bay, Fla., Chesapeake Bay, Md., and a coral surface microlayer were examined for thymidine uptake (transport), thymidine incorporation, the presence of thymidine kinase genes, and thymidine kinase enzyme activity. Of the 41 isolates tested, 37 were capable of thymidine incorporation into DNA. The four organisms that could not incorporate thymidine also transported thymidine poorly and lacked thymidine kinase activity. Attempts to detect thymidine kinase genes in the marine isolates by molecular probing with gene probes made from Escherichia coli and herpes simplex virus thymidine kinase genes proved unsuccessful. To determine if the inability to incorporate thymidine was due to the lack of thymidine kinase, one organism, Vibrio sp. strain D19, was transformed with a plasmid (pGQ3) that contained an E. coli thymidine kinase gene. Although enzyme assays indicated high levels of thymidine kinase activity in transformants, these cells still failed to incorporate exogenous thymidine into DNA or to transport thymidine into the cells. These results indicate that the inability of certain marine bacteria to incorporate thymidine may not be solely due to the lack of thymidine kinase activity but may also be due to the absence of thymidine transport systems.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck C. F., Ingraham J. L., Neuhard J., Thomassen E. Metabolism of pyrimidines and pyrimidine nucleosides by Salmonella typhimurium. J Bacteriol. 1972 Apr;110(1):219–228. doi: 10.1128/jb.110.1.219-228.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R. T. Further Verification of the Isotope Dilution Approach for Estimating the Degree of Participation of [H]thymidine in DNA Synthesis in Studies of Aquatic Bacterial Production. Appl Environ Microbiol. 1986 Nov;52(5):1212–1214. doi: 10.1128/aem.52.5.1212-1214.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson C. A., Stewart G. J., Ingraham J. L. Thymidine salvage in Pseudomonas stutzeri and Pseudomonas aeruginosa provided by heterologous expression of Escherichia coli thymidine kinase gene. J Bacteriol. 1985 Jul;163(1):291–295. doi: 10.1128/jb.163.1.291-295.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. S., Prusoff W. H. Thymidine kinase from Escherichia coli. Methods Enzymol. 1978;51:354–360. doi: 10.1016/s0076-6879(78)51047-1. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis C. L. Uptake and incorporation of thymidine by bacterial isolates from an upwelling environment. Appl Environ Microbiol. 1989 May;55(5):1267–1272. doi: 10.1128/aem.55.5.1267-1272.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garapin A. C., Colbère-Garapin F., Cohen-Solal M., Horodniceanu F., Kourilsky P. Expression of herpes simplex virus type I thymidine kinase gene in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Feb;78(2):815–819. doi: 10.1073/pnas.78.2.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holben William E., Jansson Janet K., Chelm Barry K., Tiedje James M. DNA Probe Method for the Detection of Specific Microorganisms in the Soil Bacterial Community. Appl Environ Microbiol. 1988 Mar;54(3):703–711. doi: 10.1128/aem.54.3.703-711.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey W. H., Paul J. H. Activity measurements of planktonic microbial and microfouling communities in a eutrophic estuary. Appl Environ Microbiol. 1986 Jan;51(1):157–162. doi: 10.1128/aem.51.1.157-162.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey W. H., Paul J. H. Effect of 5-fluoro-2'-deoxyuridine on [h]thymidine incorporation by bacterioplankton in the waters of southwest Florida. Appl Environ Microbiol. 1988 Feb;54(2):331–336. doi: 10.1128/aem.54.2.331-336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffrey W. H., Paul J. H. Underestimation of DNA synthesis by [h]thymidine incorporation in marine bacteria. Appl Environ Microbiol. 1988 Dec;54(12):3165–3168. doi: 10.1128/aem.54.12.3165-3168.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Littler E., Zeuthen J., McBride A. A., Trøst Sørensen E., Powell K. L., Walsh-Arrand J. E., Arrand J. R. Identification of an Epstein-Barr virus-coded thymidine kinase. EMBO J. 1986 Aug;5(8):1959–1966. doi: 10.1002/j.1460-2075.1986.tb04450.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAZAKI R., KORNBERG A. DEOXYTHYMIDINE KINASE OF ESCHERICHIA COLI. I. PURIFICATION AND SOME PROPERTIES OF THE ENZYME. J Biol Chem. 1964 Jan;239:269–274. [PubMed] [Google Scholar]
- Paul J. H., Loeb G. I. Improved Microfouling Assay Employing a DNA-Specific Fluorochrome and Polystyrene as Substratum. Appl Environ Microbiol. 1983 Aug;46(2):338–343. doi: 10.1128/aem.46.2.338-343.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul J. H. Use of hoechst dyes 33258 and 33342 for enumeration of attached and planktonic bacteria. Appl Environ Microbiol. 1982 Apr;43(4):939–944. doi: 10.1128/aem.43.4.939-944.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard P. C., Moriarty D. J. Validity of the tritiated thymidine method for estimating bacterial growth rates: measurement of isotope dilution during DNA synthesis. Appl Environ Microbiol. 1984 Dec;48(6):1076–1083. doi: 10.1128/aem.48.6.1076-1083.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robarts R. D., Wicks R. J., Sephton L. M. Spatial and Temporal Variations in Bacterial Macromolecule Labeling with [methyl-H]Thymidine in a Hypertrophic Lake. Appl Environ Microbiol. 1986 Dec;52(6):1368–1373. doi: 10.1128/aem.52.6.1368-1373.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Tomioka H., Ohkido S. Further studies on thymidine kinase: distribution pattern of the enzyme in bacteria. J Gen Microbiol. 1985 Nov;131(11):3091–3098. doi: 10.1099/00221287-131-11-3091. [DOI] [PubMed] [Google Scholar]
- Upton C., McFadden G. Identification and nucleotide sequence of the thymidine kinase gene of Shope fibroma virus. J Virol. 1986 Dec;60(3):920–927. doi: 10.1128/jvi.60.3.920-927.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Berger S. L., Kimmel A. R. Molecular hybridization of immobilized nucleic acids: theoretical concepts and practical considerations. Methods Enzymol. 1987;152:399–407. doi: 10.1016/0076-6879(87)52046-8. [DOI] [PubMed] [Google Scholar]




