Abstract
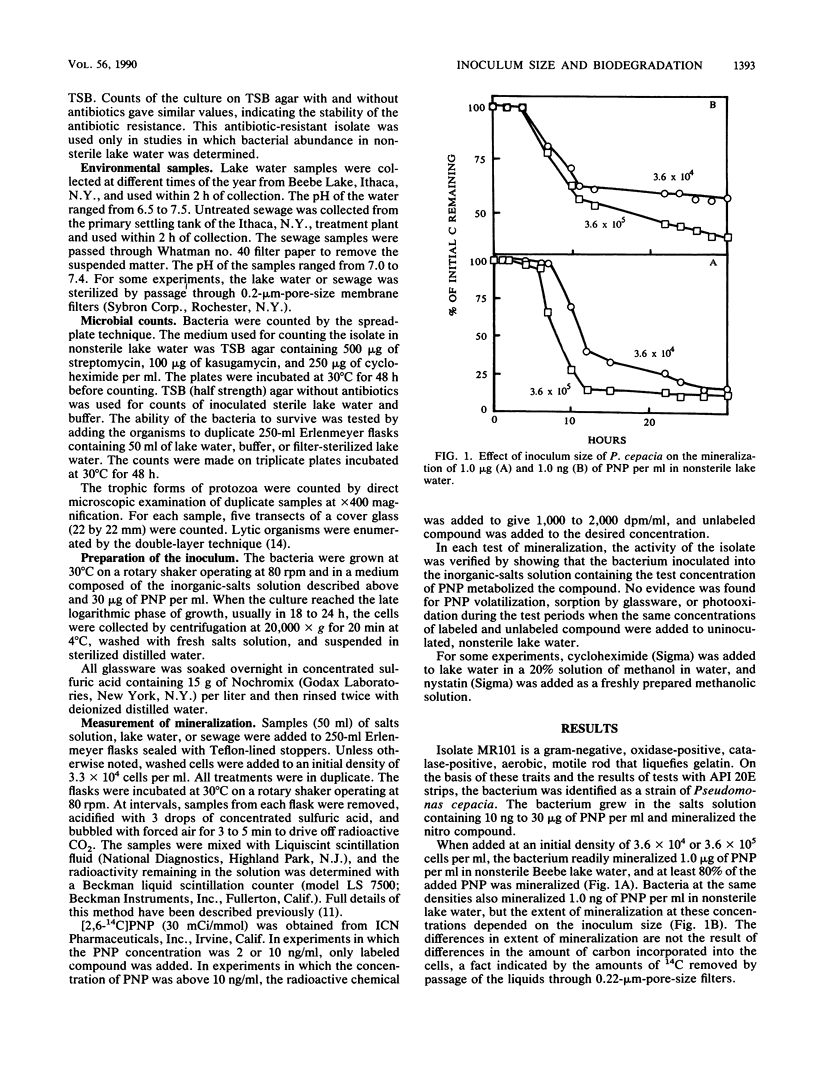
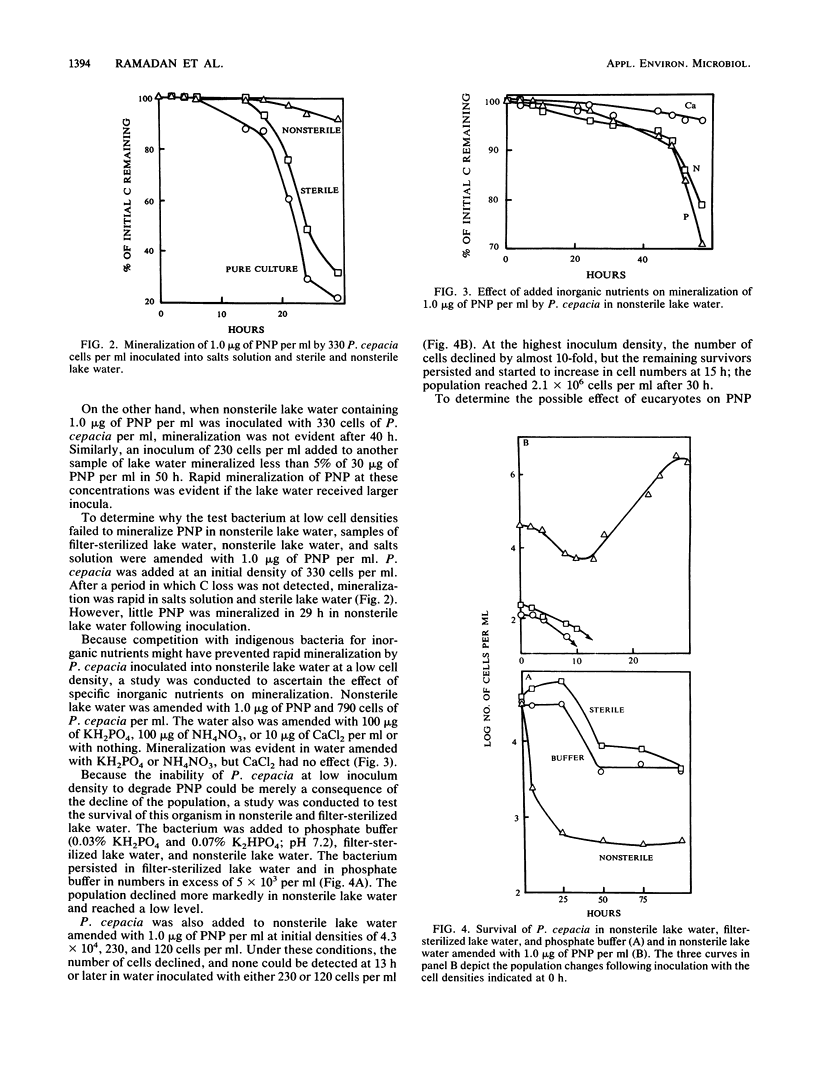
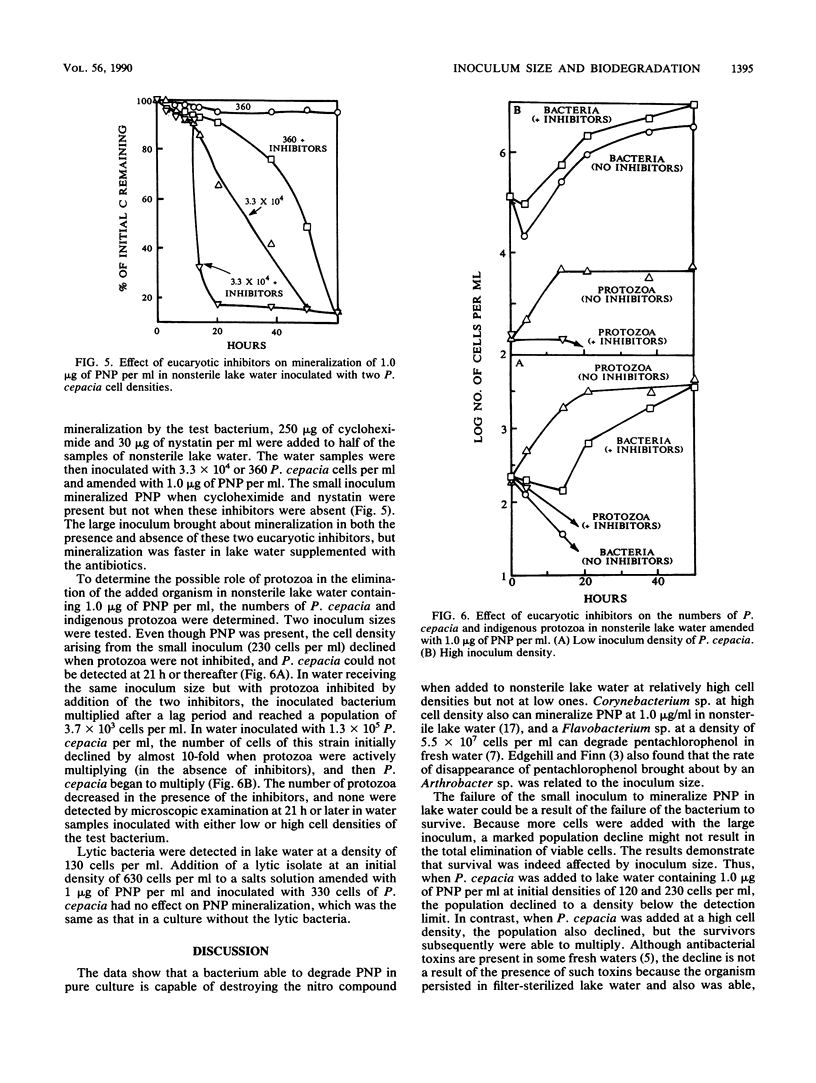
A study was conducted to determine the role of inoculum size of a bacterium introduced into nonsterile lake water in the biodegradation of a synthetic chemical. The test species was a strain of Pseudomonas cepacia able to grow on and mineralize 10 ng to 30 micrograms of p-nitrophenol (PNP) per ml in salts solution. When introduced into water from Beebe Lake at densities of 330 cells per ml, P. cepacia did not mineralize 1.0 microgram of PNP per ml. However, PNP was mineralized in lake water inoculated with 3.3 X 10(4) to 3.6 X 10(5) P. cepacia cells per ml. In lake water containing 1.0 microgram of PNP per ml, a P. cepacia population of 230 or 120 cells per ml declined until no cells were detectable at 13 h, but when the initial density was 4.3 X 10(4) cells per ml, sufficient survivors remained after the initial decline to multiply at the expense of PNP. The decline in bacterial abundance coincided with multiplication of protozoa. Cycloheximide and nystatin killed the protozoa and allowed the bacterium to multiply and mineralize 1.0 microgram of PNP, even when the initial P. cepacia density was 230 or 360 cells per ml. The lake water contained few lytic bacteria. The addition of KH2PO4 or NH4NO3 permitted biodegradation of PNP at low cell densities of P. cepacia. We suggest that a species able to degrade a synthetic chemical in culture may fail to bring about the same transformation in natural waters, because small populations added as inocula may be eliminated by protozoan grazing or may fail to survive because of nutrient deficiencies.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barles R. W., Daughton C. G., Hsieh D. P. Accelerated parathion degradation in soil inoculated with acclimated bacteria under field conditions. Arch Environ Contam Toxicol. 1979;8(6):647–660. doi: 10.1007/BF01054867. [DOI] [PubMed] [Google Scholar]
- Edgehill R. U., Finn R. K. Microbial treatment of soil to remove pentachlorophenol. Appl Environ Microbiol. 1983 Mar;45(3):1122–1125. doi: 10.1128/aem.45.3.1122-1125.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein R. M., Mallory L. M., Alexander M. Reasons for possible failure of inoculation to enhance biodegradation. Appl Environ Microbiol. 1985 Oct;50(4):977–983. doi: 10.1128/aem.50.4.977-983.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein T. M., Alexander M. Bacterial inhibitors in lake water. Appl Environ Microbiol. 1986 Jul;52(1):114–118. doi: 10.1128/aem.52.1.114-118.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirkot C. K., Gupta K. G. Accelerated tetramethylthiuram disulfide (TMTD) degradation in soil by inoculation with TMTD-utilizing bacteria. Bull Environ Contam Toxicol. 1985 Sep;35(3):354–361. doi: 10.1007/BF01636522. [DOI] [PubMed] [Google Scholar]
- Subba-Rao R. V., Rubin H. E., Alexander M. Kinetics and extent of mineralization of organic chemicals at trace levels in freshwater and sewage. Appl Environ Microbiol. 1982 May;43(5):1139–1150. doi: 10.1128/aem.43.5.1139-1150.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindoll C. M., Aelion C. M., Pfaender F. K. Influence of inorganic and organic nutrients on aerobic biodegradation and on the adaptation response of subsurface microbial communities. Appl Environ Microbiol. 1988 Jan;54(1):212–217. doi: 10.1128/aem.54.1.212-217.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins B. A., Jones S. H., Alexander M. Explanations for the acclimation period preceding the mineralization of organic chemicals in aquatic environments. Appl Environ Microbiol. 1987 Apr;53(4):791–796. doi: 10.1128/aem.53.4.791-796.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]



