Abstract
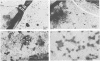
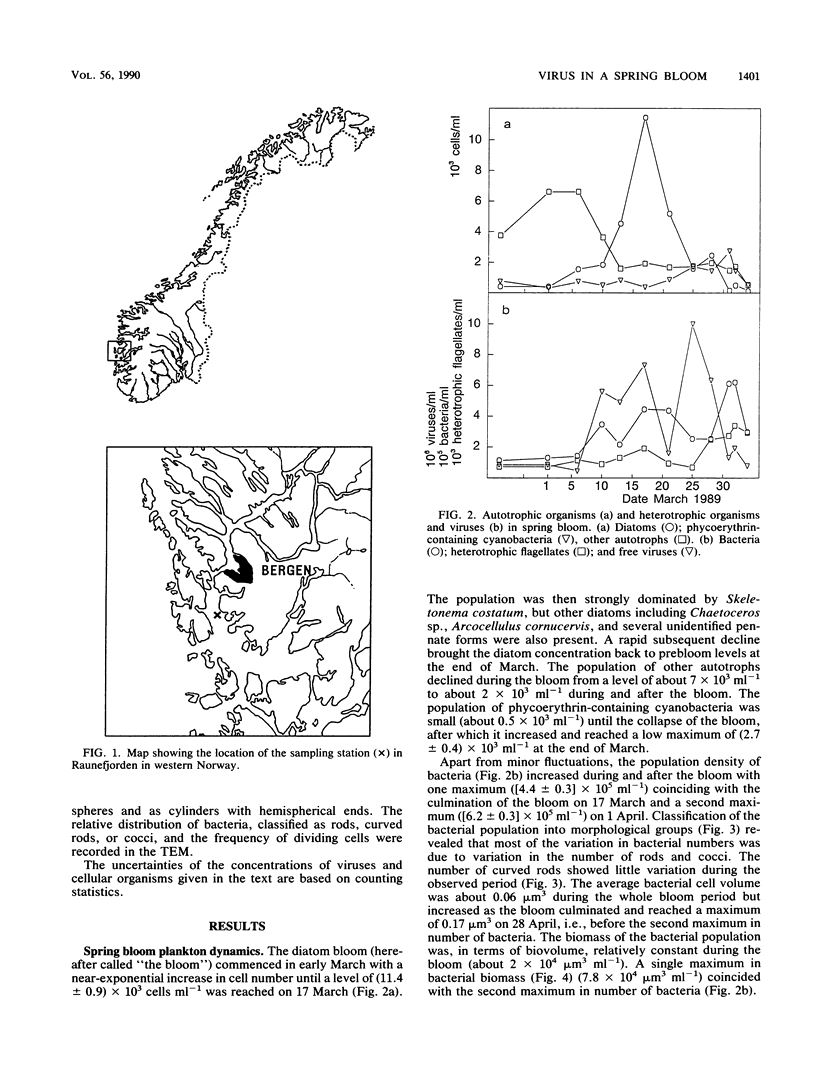
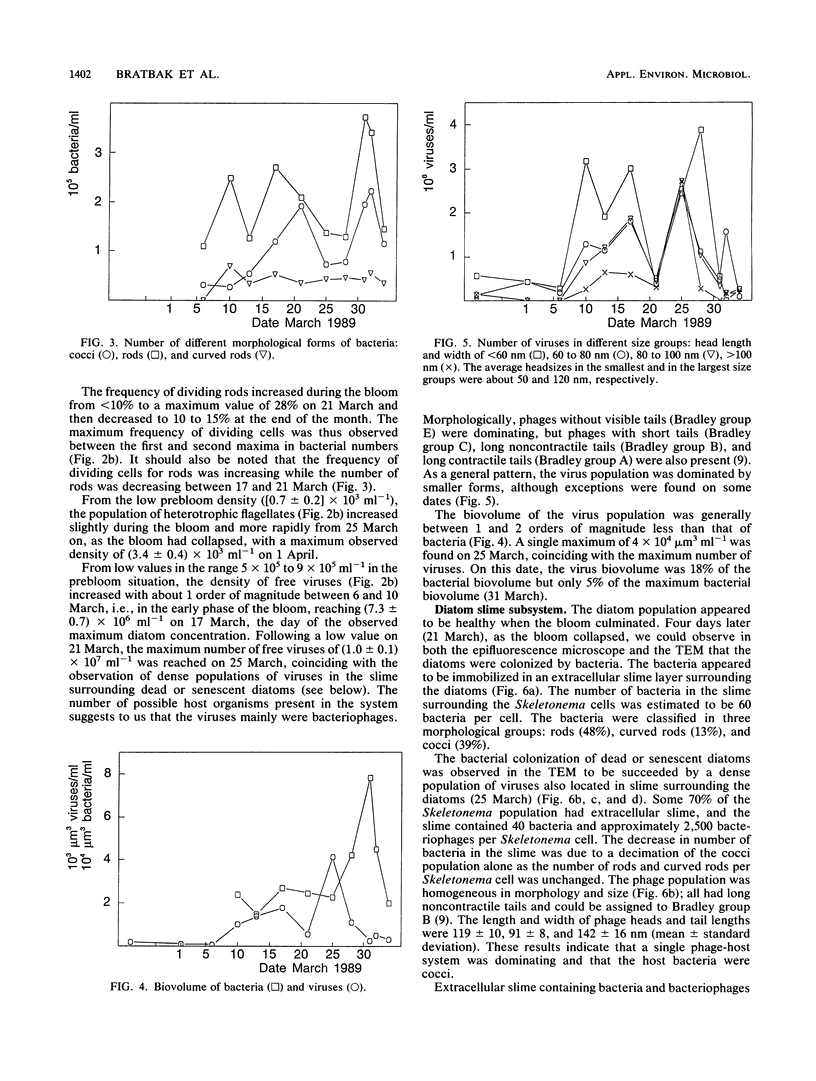
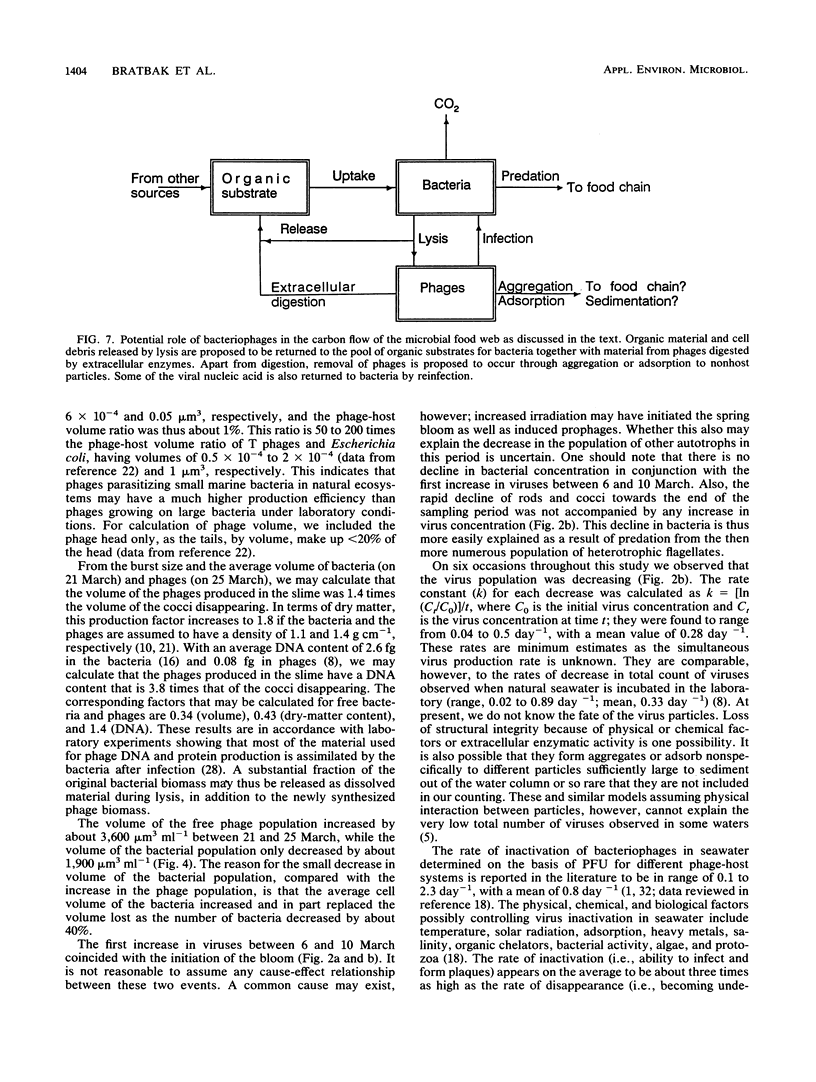
Population sizes of algae, bacteria, heterotrophic flagellates, and viruses were observed through the 1989 spring diatom bloom in Raunefjorden in western Norway. The culmination of the diatom bloom was followed by a peak in the concentration of bacteria and an increase in the concentration of heterotrophic flagellates, a pattern consistent with the concept of a food chain from photosynthetically produced organic material, through bacteria, to bacterivorous flagellates. The concentration of viruses varied through the spring bloom from 5 × 105 in the prebloom situation to a maximum of 1.3 × 107 viruses ml−1 1 week after the peak of the diatom bloom. Coinciding with the collapse in the diatom bloom, a succession of bacteria and viruses was observed in the mucous layer surrounding dead or senescent diatoms, with an estimated maximum of 23% of the total virus population attached to the diatoms. The dynamic behavior observed for the virus population rules out the possibility that it is dominated by inactive species, and the viruses are suggested to be active members of the microbial food web as agents causing lysis in parts of the bacterial population, diverting part of the bacterial production from the predatory food chain.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albright L. J., McCrae S. K. Annual bacterioplankton biomasses and productivities in a temperate west coast canadian fjord. Appl Environ Microbiol. 1987 Jun;53(6):1277–1285. doi: 10.1128/aem.53.6.1277-1285.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergh O., Børsheim K. Y., Bratbak G., Heldal M. High abundance of viruses found in aquatic environments. Nature. 1989 Aug 10;340(6233):467–468. doi: 10.1038/340467a0. [DOI] [PubMed] [Google Scholar]
- Bloem J., Bär-Gilissen M. J., Cappenberg T. E. Fixation, counting, and manipulation of heterotrophic nanoflagellates. Appl Environ Microbiol. 1986 Dec;52(6):1266–1272. doi: 10.1128/aem.52.6.1266-1272.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley D. E. Ultrastructure of bacteriophage and bacteriocins. Bacteriol Rev. 1967 Dec;31(4):230–314. doi: 10.1128/br.31.4.230-314.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bratbak G., Dundas I. Bacterial dry matter content and biomass estimations. Appl Environ Microbiol. 1984 Oct;48(4):755–757. doi: 10.1128/aem.48.4.755-757.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Børsheim K. Y., Bratbak G., Heldal M. Enumeration and biomass estimation of planktonic bacteria and viruses by transmission electron microscopy. Appl Environ Microbiol. 1990 Feb;56(2):352–356. doi: 10.1128/aem.56.2.352-356.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caron D. A. Technique for enumeration of heterotrophic and phototrophic nanoplankton, using epifluorescence microscopy, and comparison with other procedures. Appl Environ Microbiol. 1983 Aug;46(2):491–498. doi: 10.1128/aem.46.2.491-498.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducklow H. W., Purdie D. A., Williams P. J., Davies J. M. Bacterioplankton: a sink for carbon in a coastal marine plankton community. Science. 1986 May 16;232(4752):865–867. doi: 10.1126/science.232.4752.865. [DOI] [PubMed] [Google Scholar]
- Torrella F., Morita R. Y. Evidence by electron micrographs for a high incidence of bacteriophage particles in the waters of Yaquina Bay, oregon: ecological and taxonomical implications. Appl Environ Microbiol. 1979 Apr;37(4):774–778. doi: 10.1128/aem.37.4.774-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zachary A. An ecological study of bacteriophages of Vibrio natriegens. Can J Microbiol. 1978 Mar;24(3):321–324. doi: 10.1139/m78-053. [DOI] [PubMed] [Google Scholar]
- Zachary A. Physiology and ecology of bacteriophages of the marine bacterium Beneckea natriegens: salinity. Appl Environ Microbiol. 1976 Mar;31(3):415–422. doi: 10.1128/aem.31.3.415-422.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]