Abstract
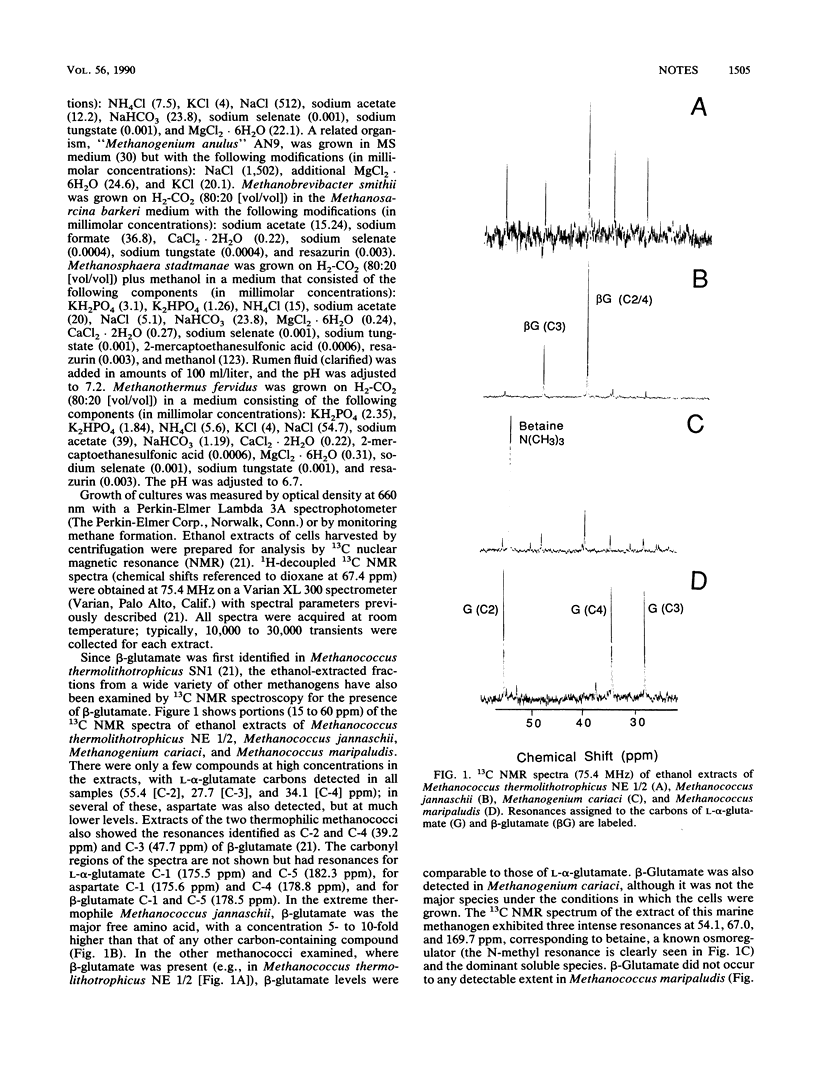
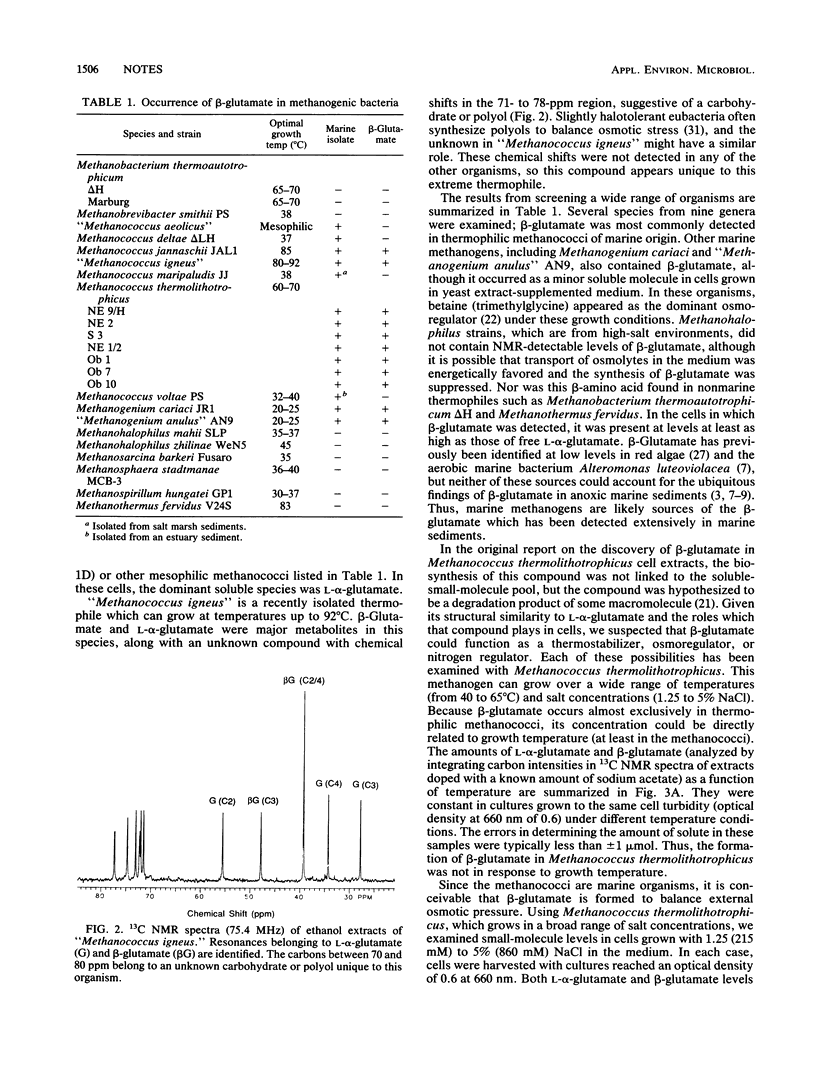
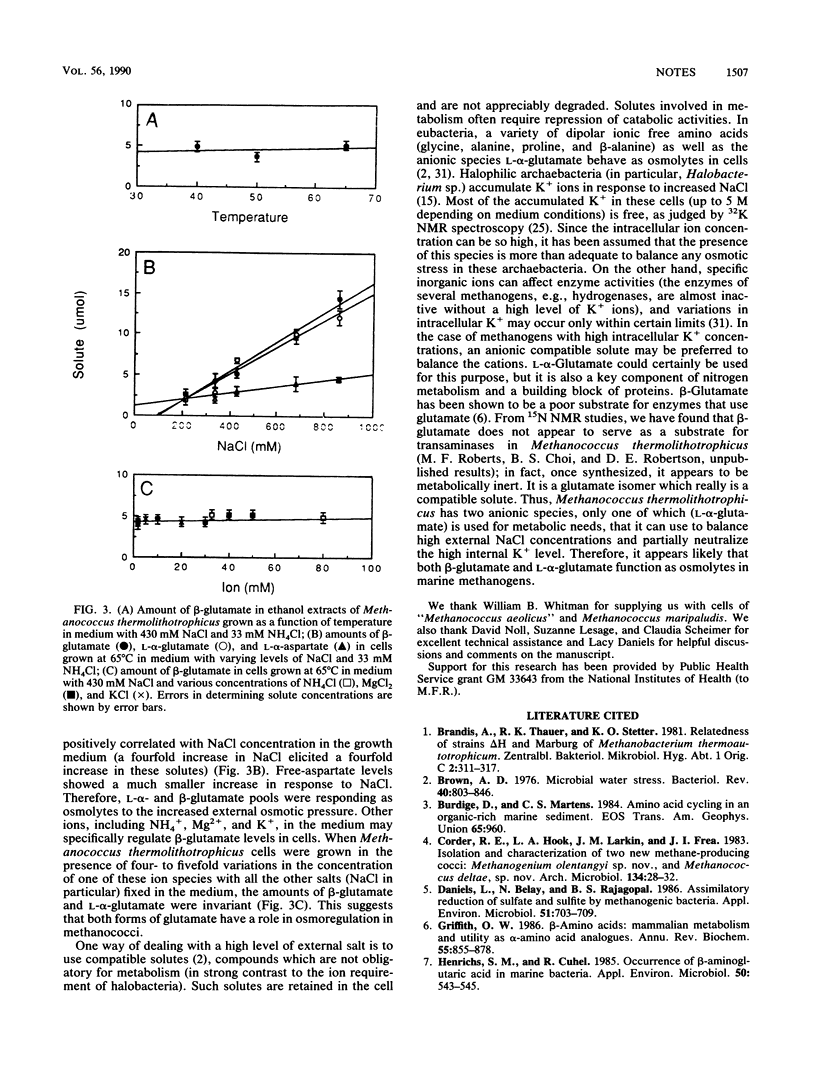
The unusual compound beta-aminoglutaric acid (beta-glutamate) has been identified by 13C nuclear magnetic resonance spectroscopy in soluble extracts of marine methanogenic bacteria. We examined several methanogen species representing nine genera and found that beta-glutamate occurred in methanococci and two methanogenium strains (Methanogenium cariaci JR1 and "Methanogenium anulus" AN9). The presence of this compound in the methanococci examined was further restricted to thermophilic members of the genus Methanococcus, including Methanococcus thermolithotrophicus strains, Methanococcus jannaschii, and "Methanococcus igneus." The two Methanogenium strains examined were mesophiles. Studies using Methanococcus thermolithotrophicus showed that levels of beta-glutamate in cells of that species were not affected by variation in growth temperature (40 to 65 degrees C), NH4+ (2 to 80 mM), Mg2+ (10 to 50 mM), or K+ (2 to 10 mM) in the medium. In contrast, soluble pools of beta-glutamate and L-alpha-glutamate (the other major free amino acid in all the methanococci) were proportional to NaCl levels in the growth medium. This dependence of beta-glutamate and L-alpha-glutamate concentrations on salt levels in the medium suggests that they function as osmolytes in these cells.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown A. D. Microbial water stress. Bacteriol Rev. 1976 Dec;40(4):803–846. doi: 10.1128/br.40.4.803-846.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels L., Belay N., Rajagopal B. S. Assimilatory reduction of sulfate and sulfite by methanogenic bacteria. Appl Environ Microbiol. 1986 Apr;51(4):703–709. doi: 10.1128/aem.51.4.703-709.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith O. W. Beta-amino acids: mammalian metabolism and utility as alpha-amino acid analogues. Annu Rev Biochem. 1986;55:855–878. doi: 10.1146/annurev.bi.55.070186.004231. [DOI] [PubMed] [Google Scholar]
- Henrichs S. M., Cuhel R. Occurrence of beta-Aminoglutaric Acid in Marine Bacteria. Appl Environ Microbiol. 1985 Aug;50(2):543–545. doi: 10.1128/aem.50.2.543-545.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hippe H., Caspari D., Fiebig K., Gottschalk G. Utilization of trimethylamine and other N-methyl compounds for growth and methane formation by Methanosarcina barkeri. Proc Natl Acad Sci U S A. 1979 Jan;76(1):494–498. doi: 10.1073/pnas.76.1.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanyi J. K. Salt-dependent properties of proteins from extremely halophilic bacteria. Bacteriol Rev. 1974 Sep;38(3):272–290. doi: 10.1128/br.38.3.272-290.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathrani I. M., Boone D. R., Mah R. A., Fox G. E., Lau P. P. Methanohalophilus zhilinae sp. nov., an alkaliphilic, halophilic, methylotrophic methanogen. Int J Syst Bacteriol. 1988 Apr;38(2):139–142. doi: 10.1099/00207713-38-2-139. [DOI] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J., Conway de Macario E., Macario A. J. Isolation of Methanobrevibacter smithii from human feces. Appl Environ Microbiol. 1982 Jan;43(1):227–232. doi: 10.1128/aem.43.1.227-232.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. L., Wolin M. J. Methanosphaera stadtmaniae gen. nov., sp. nov.: a species that forms methane by reducing methanol with hydrogen. Arch Microbiol. 1985 Mar;141(2):116–122. doi: 10.1007/BF00423270. [DOI] [PubMed] [Google Scholar]
- Patel G. B., Roth L. A., van den Berg L., Clark D. S. Characterization of a strain of Methanospirillum hungatti. Can J Microbiol. 1976 Sep;22(9):1404–1410. doi: 10.1139/m76-208. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Lesage S., Roberts M. F. Beta-aminoglutaric acid is a major soluble component of Methanococcus thermolithotrophicus. Biochim Biophys Acta. 1989 Sep 15;992(3):320–326. doi: 10.1016/0304-4165(89)90091-3. [DOI] [PubMed] [Google Scholar]
- Robertson D. E., Noll D., Roberts M. F., Menaia J. A., Boone D. R. Detection of the osmoregulator betaine in methanogens. Appl Environ Microbiol. 1990 Feb;56(2):563–565. doi: 10.1128/aem.56.2.563-565.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid K., Thomm M., Laminet A., Laue F. G., Kessler C., Stetter K. O., Schmitt R. Three new restriction endonucleases MaeI, MaeII and MaeIII from Methanococcus aeolicus. Nucleic Acids Res. 1984 Mar 26;12(6):2619–2628. doi: 10.1093/nar/12.6.2619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shporer M., Civan M. M. Pulsed nuclear magnetic resonance study of 39K within halobacteria. J Membr Biol. 1977 May 12;33(3-4):385–400. doi: 10.1007/BF01869525. [DOI] [PubMed] [Google Scholar]
- TAKEMOTO T., SAI T. [STUDIES ON THE CONSTITUENTS OF CHONDRIA ARMATA. VII. ISOLATION OF 3-AMINOGLUTARIC ACID]. Yakugaku Zasshi. 1965 Jan;85:33–37. [PubMed] [Google Scholar]
- WOLIN E. A., WOLIN M. J., WOLFE R. S. FORMATION OF METHANE BY BACTERIAL EXTRACTS. J Biol Chem. 1963 Aug;238:2882–2886. [PubMed] [Google Scholar]
- Whitman W. B., Ankwanda E., Wolfe R. S. Nutrition and carbon metabolism of Methanococcus voltae. J Bacteriol. 1982 Mar;149(3):852–863. doi: 10.1128/jb.149.3.852-863.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xun L., Boone D. R., Mah R. A. Control of the Life Cycle of Methanosarcina mazei S-6 by Manipulation of Growth Conditions. Appl Environ Microbiol. 1988 Aug;54(8):2064–2068. doi: 10.1128/aem.54.8.2064-2068.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey P. H., Clark M. E., Hand S. C., Bowlus R. D., Somero G. N. Living with water stress: evolution of osmolyte systems. Science. 1982 Sep 24;217(4566):1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- Zeikus J. G., Wolfe R. S. Methanobacterium thermoautotrophicus sp. n., an anaerobic, autotrophic, extreme thermophile. J Bacteriol. 1972 Feb;109(2):707–715. doi: 10.1128/jb.109.2.707-713.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



