Abstract
Background
Solitary extramedullary plasmacytoma of the parotid gland is a rare condition. Intracytoplasmic Crystalline inclusions in the tumor are even rarer and have been reported only once in the parotid gland.
Case presentation
We report here, a case of plasmacytoma of the parotid gland with intracellular crystalline inclusions in a 73-year-old woman
Conclusion
Solitary extramedullary plasmacytoma of the parotid gland and crystalline inclusions in the tumor is of rare occurrence. The importance of such a finding with relation to tumor progression, clinical course of the disease or prognosis in general remains to be understood.
Keywords: Parotid, neoplasm, plasmocytoma, inclusion, salivary gland.
Introduction
Plasmacytoma is traditionally divided into medullary and extramedullary type, which in turn could be either solitary or multiple in distribution. The most common form of plasmacytoma is the generalized medullary form that is known as multiple myeloma Solitary primary extramedullary plasmacytoma is a neoplasm of the plasma cells arising in regions other than bone marrow in patients with no clinical or biochemical evidence of multiple myeloma. Booth et al [1] in their review of 250 reported cases stated that primary extramedullary plasmacytoma usually affect the head and neck area. Involvement of the parotid gland is extremely rare and since the first case in 1965 [2] there are only a few documented cases in the published literature [2-15] as listed in Table 1. Although review of the literature shows a favorable prognosis, clinical behavior of this disease is not well explained. The purpose of this report is to add another case of this kind, not only because of its rarity, but also because of its rare and interesting finding of intracellular crystalline inclusions.
Table 1.
Review-published Cases of Parotid Plasmacytomas
| Author | Sex/Age | Treatment |
| 1-Ustun MO et al. [15] | F/77 | S+R |
| 2-Hari CK, Roblin DG. [14] | F/77 | S |
| 3-Gonzalez-Garcia J et al. [13] | M/63 | S+R |
| 4-El-Naggar AK [4] | M/73 | S |
| 5-Kerr PD, Dort JC [12] | F/73 | S+R |
| 6-Rothfield RE et al. [10] | M/53 | S |
| 7-Simi U et al. [9] | M/58 | S |
| 8-Scholl P, Jafek BW [8] | F/60 | S |
| 9-Ebbers J [7] | M/68 | R |
| 10-Kanoh et al. [6] | F/78 | S+R |
| 11-Edney JA et al [5] | M/38 | S |
| 12-Ferlito A et al [4] | M/47 | S+R+Ch |
| 13-Pahor AL. [3] | F/61 M/69 | S+Ch R |
| 14-Vainio-Mattila J [2] | F/74 | S+R |
S = Surgery R = Radio therapy Ch = Chemo therapy
Case Presentation
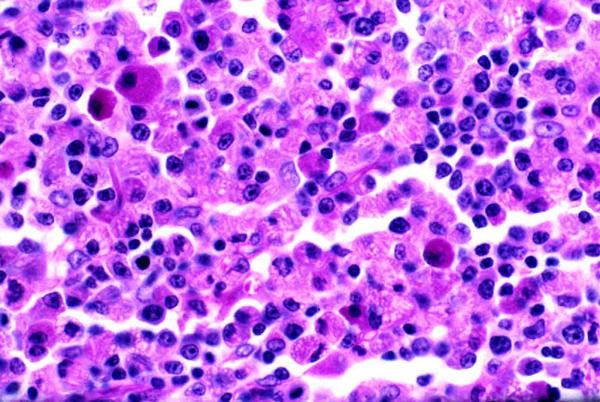
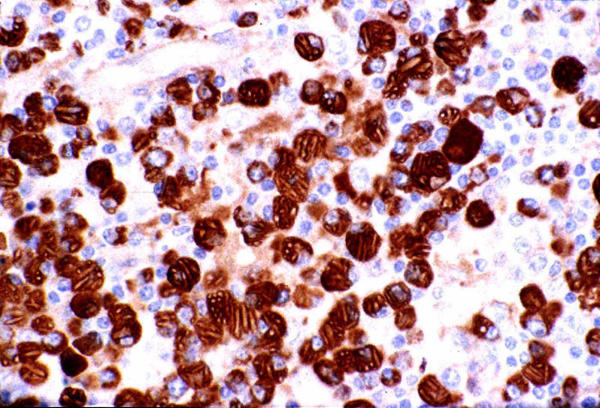
A 73-year-old Caucasian female was investigated at the Royal University Hospital of Saskatoon, Canada, for a non-tender mobile mass at the angle of the right mandible with a history of recent rapid growth over the past two months. Clinical examination was otherwise non-contributory. Past medical history included psoriasis, hypertension and osteoarthritis of both hip joints and treated Hodgkin's lymphoma 10 years back. Cytology of the fine needle aspiration of the parotid mass showed numerous polymorphic lymphocytes (Figure 1). Subsequently a superficial parotidectomy with conservation of the facial nerve was performed. Histopathological examination of the excised mass at initial frozen section analysis revealed the parotid gland to be completely replaced by sheets of lesional cells that were non-salivary in origin. Detailed pathological analysis showed sheets of plasma cells and plasmacytoid cells with a fair number of the plasma cells and histiocytes containing crystalline inclusion bodies many of which were linearly profiled and of multiple shapes (Figure 2, 3). Multiple cytoplasmic globules (Russell bodies), some nuclear inclusion like structures (Dutcher bodies), mild nuclear atypia and rare mitosis were also observed. Immuno-histochemical staining showed the plasma cells to be monoclonal (kappa restricted) and the small lymphoid cells in the background to be predominantly T-cells (CD45RO+), along with a smaller proportion of B-cells (CD20+) (Figure 4). A complete multiple myeloma work-up, including bone marrow biopsy, total body skeletal survey, immunoelectrophoresis, quantitative immunoglobulins and urinary Bence Jones proteins remained negative. She developed local recurrence of her disease 18 months and 30 months later, which was managed by both radiotherapy and chemotherapy. Her clinical follow up for two years has remained uneventful.
Figure 1.
Fine needle aspiration cytology of the right parotid mass showing numerous polymorphic lymphocytes. (medium high power × 550; Diff Quick stain).
Figure 2.
Crystalline inclusion bodies in tissue section with PAS stain Periodic-Acid-Schiff Staining (medium power × 250) reveals sheets of neoplastic plasma cells with multiple intracytoplasmic crystalline inclusion bodies both within the plasma cells and the adjacent histiocytes.
Figure 3.
Electron microscopic analysis of crystalline inclusions. Ultrastructural analysis of these crystalline inclusions is demonstrated (scale 1.70 cm = 300 nm at × 5675)
Figure 4.
Immunohistochemical analysis Immunohistochemical staining shows the plasma cells to be IgG positive with kappa restriction pattern (medium power × 250)
Discussion
Solitary primary extramedullary plasmacytomas are rare and found principally in elderly people. They occur predominantly in the head and neck area with a tendency to involve the submucosal tissues of the upper airway accounting for 0.5% of all upper respiratory tract neoplasms. In two third of the cases, these neoplasms are located in the upper respiratory tract or oral cavity primarily in the nose, sinuses and nasopharynx [1].
Histopathological analysis alone is not sufficient in order to make a diagnosis of primary extramedullary plasmacytoma as multiple myeloma must be excluded by a through skeletal and marrow workup, immuno-electrophoresis and quantitative immunoglobulins with absent urinary Bence Jones proteins. Fine needle aspiration cytology of plasmacytoma of the parotid gland is often reported unremarkable as in our case.
Treatment of solitary extramedullary plasmacytoma consists primarily of eradication of the local lesion and in this context surgery seems to be the primary line of treatment. Radiation therapy either alone in cases deemed unsuitable for surgery or as an adjunct with surgical removal has also been used. Local recurrences are also usually treated by radiation therapy [16] Although not proven, chemotherapy is theoretically advantageous, both in enhancing local control and in potentially eradicating sub-clinical disease to delay or prevent the development of myeloma. The patterns of relapse of primary extramedullary plasmacytoma include transformation to multiple myeloma, metastasis and local recurrence. Recurrences are usually local and respond well to radiation therapy. Combined chemotherapy and radiation therapy is recommended in high-risk patients to increase the local control rate and cure rate [16] In our case radiotherapy could have been added after surgery in order to eradicate residual tumor and to prevent local relapse. However, the validity of such adjuvant therapy with complete excision of the tumor with clear margins remains contradictory. Radiotherapy was administered in our case, upon local recurrence. The second local recurrence was managed by chemotherapy.
Crystalline inclusions composed of monoclonal immunoglobulins have been reported in multiple myeloma and lymphoproliferative disorders [17]. A single publication of these crystalline inclusions in primary extramedullary plasmacytoma of the parotid gland has been reported by el-Naggar et al. [11] The crystals may be rectangular or square in shape, finely uniform or globular. Crystals can be stained with anti light and/or anti heavy chains of globulin, which often manifest a light chain restriction. They can be found in histiocytes, which absorb the immunoglobulins [17]. The crystalline inclusions in our case were positive for IGg with Kappa restriction, confirming the intracellular crystallization of the immunoglobulin. Electron micrographs of the crystalline deposits show the polygonal, rectangular or diamond shape of this finding in solitary plasmacytoma. The importance of such a finding with relation to tumor progression, clinical course of the disease or prognosis in general remains uncertain.
Conclusions
Primary solitary extramedullary plasmacytoma of the parotid gland is of rare occurrence. Accurate diagnosis by fine needle aspiration requires a high index of suspicion. The presence of intracytoplasmic crystalline inclusions can further add confusion to the accurate pathological diagnosis of this entity especially at frozen section analysis.
Conflict of interest
None declared.
Author's contributions
RK is the surgical pathologist who diagnosed and followed-up this case and BT participated actively in the production of this manuscript.
Acknowledgments
Acknowledgements
The authors would like to thank Mr Todd Reichert and Michelle Hesson for their expert technical assistance in the production of the illustrations. Written consent was obtained from the patient for the use of all clinical material for research and publication.
Contributor Information
Rani Kanthan, Email: inarkanth@shaw.ca.
Bahman Torkian, Email: torkian@yahoo.com.
References
- Booth JB, Cheesman AD, Vincenti NH. Extramedullary plasmacytomata of the upper respiratory tract. Ann Otol Rhinol Laryngol. 1973;82:709–715. doi: 10.1177/000348947308200515. [DOI] [PubMed] [Google Scholar]
- Vainio-Mattila J. Plasmacytoma of the parotid gland. Arch Otolaryngol. 1965;82:635–637. doi: 10.1001/archotol.1965.00760010637016. [DOI] [PubMed] [Google Scholar]
- Pahor AL. Extramedullary plasmacytoma of the head and neck, parotid and submandibular salivary glands. J Laryngol Otol. 1977;91:241–258. doi: 10.1017/s0022215100083626. [DOI] [PubMed] [Google Scholar]
- Ferlito A, Polidoro F, Recher G. Extramedullary plasmacytoma of the parotid gland. Laryngoscope. 1980;90:486–493. doi: 10.1002/lary.5540900316. [DOI] [PubMed] [Google Scholar]
- Edney JA, Thompson JS, Conley MC, Moore GE. Plasmacytoma of the parotid gland. J Surg Oncol. 1985;28:165–167. doi: 10.1002/jso.2930280303. [DOI] [PubMed] [Google Scholar]
- Kanoh T, Hattori N, Uchino H, Fujita A, Ohmura M, Makimoto K. Extramedullary plasmacytoma of the parotid gland: report of a case and review of the literature. Tohoku J Exp Med. 1985;146:469–478. doi: 10.1620/tjem.146.469. [DOI] [PubMed] [Google Scholar]
- Ebbers J. [Plasmacytoma of the parotid gland] Laryngol Rhinol Otol (Stuttg) 1986;65:127–129. [PubMed] [Google Scholar]
- Scholl P, Jafek BW. Extramedullary plasmacytoma of the parotid gland. Ear Nose Throat J. 1986;65:564–567. [PubMed] [Google Scholar]
- Simi U, Marchetti G, Bruno R, Di Nasso F, Cardini M. Plasmacytoma of the parotid gland. Report of a case and review of the world literature. Acta Otorhinolaryngol Belg. 1988;42:93–96. [PubMed] [Google Scholar]
- Rothfield RE, Johnson JT, Stavrides A. Extramedullary plasmacytoma of the parotid. Head Neck. 1990;12:352–354. doi: 10.1002/hed.2880120414. [DOI] [PubMed] [Google Scholar]
- el-Naggar AK, Ordonez NG, Batsakis JG. Parotid gland plasmacytoma with crystalline deposits. Oral Surg Oral Med Oral Pathol. 1991;71:206–208. doi: 10.1016/0030-4220(91)90470-w. [DOI] [PubMed] [Google Scholar]
- Kerr PD, Dort JC. Primary extramedullary plasmacytoma of the salivary glands. J Laryngol Otol. 1991;105:687–692. doi: 10.1017/s0022215100117049. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Garcia J, Ghufoor K, Sandhu G, Thorpe PA, Hadley J. Primary extramedullary plasmacytoma of the parotid gland: a case report and review of the literature. J Laryngol Otol. 1998;112:179–181. doi: 10.1017/s0022215100140253. [DOI] [PubMed] [Google Scholar]
- Hari CK, Roblin DG. Solitary plasmacytoma of the parotid gland. Int J Clin Pract. 2000;54:197–198. [PubMed] [Google Scholar]
- Ustun MO, Ekinci N, Payzin B. Extramedullary plasmacytoma of the parotid gland. Report of a case with extensive amyloid deposition masking the cytologic and histopathologic picture. Acta Cytol. 2001;45:449–453. doi: 10.1159/000327648. [DOI] [PubMed] [Google Scholar]
- Tsang RW, Gospodarowicz MK, Pintilie M, Bezjak A, Wells W, Hodgson DC, Stewart AK. Solitary plasmacytoma treated with radiotherapy: impact of tumor size on outcome. Int J Radiat Oncol Biol Phys. 2001;50:113–120. doi: 10.1016/S0360-3016(00)01572-8. [DOI] [PubMed] [Google Scholar]
- Suarez P, el-Naggar AK, Batsakis JG. Intracellular crystalline deposits in lymphoplasmacellular disorders. Ann Otol Rhinol Laryngol. 1997;106:170–172. doi: 10.1177/000348949710600215. [DOI] [PubMed] [Google Scholar]