Abstract
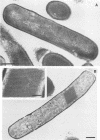
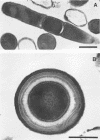
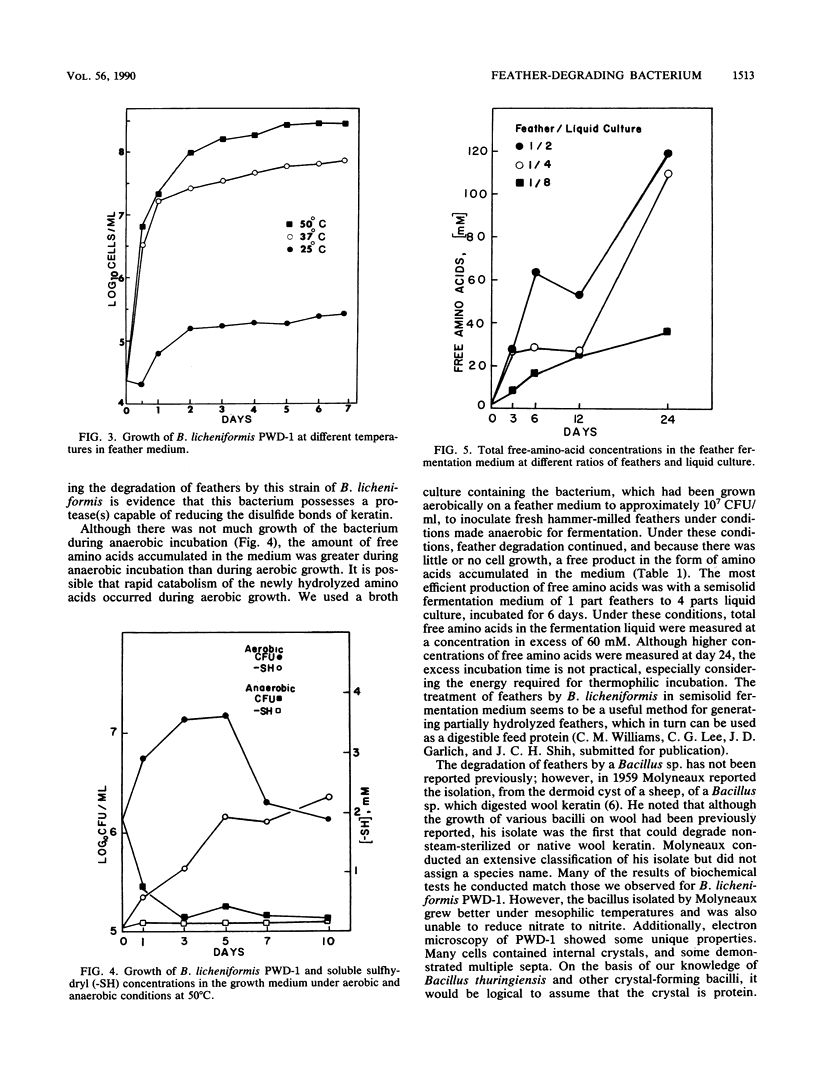
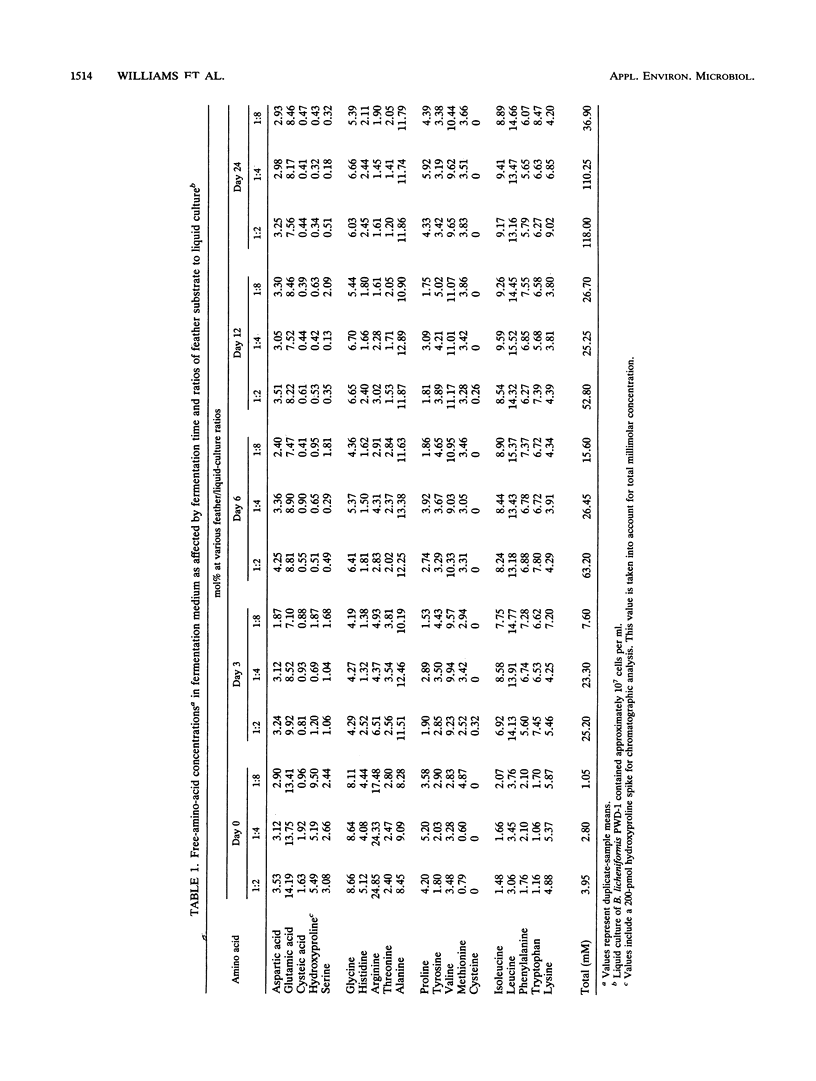
A feather-degrading culture was enriched with isolates from a poultry waste digestor and adapted to grow with feathers as its primary source of carbon, sulfur, and energy. Subsequently, a feather-hydrolytic, endospore-forming, motile, rod-shaped bacterium was isolated from the feather-degrading culture. The organism was Gram stain variable and catalase positive and demonstrated facultative growth at thermophilic temperatures. The optimum rate of growth in nutrient broth occurred at 45 to 50°C and at pH 7.5. Electron microscopy of the isolate showed internal crystals. The microorganism was identified as Bacillus licheniformis PWD-1. Growth on hammer-milled-feather medium of various substrate concentrations was determined by plate colony count. Maximum growth (approximately 109 cells per ml) at 50°C occurred 5 days postinoculation on 1% feather substrate. Feather hydrolysis was evidenced as free amino acids produced in the medium. The most efficient conditions for feather fermentation occurred during the incubation of 1 part feathers to 2 parts B. licheniformis PWD-1 culture (107 cells per ml) for 6 days at 50°C. These data indicate a potential biotechnique for degradation and utilization of feather keratin.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Elmayergi H. H., Smith R. E. Influence of growth of Streptomyces fradiae on pepsin-HCl digestibility and methionine content of feather meal. Can J Microbiol. 1971 Aug;17(8):1067–1072. doi: 10.1139/m71-169. [DOI] [PubMed] [Google Scholar]
- NICKERSON W. J., NOVAL J. J., ROBISON R. S. KERATINASE. I. PROPERTIES OF THE ENZYME CONJUGATED ELABORATED BY STREPTOMYCES FRADIAE. Biochim Biophys Acta. 1963 Sep 3;77:73–86. doi: 10.1016/0006-3002(63)90470-0. [DOI] [PubMed] [Google Scholar]
- NOVAL J. J., NICKERSON W. J. Decomposition of native keratin by Streptomyces fradiae. J Bacteriol. 1959 Mar;77(3):251–263. doi: 10.1128/jb.77.3.251-263.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih J. C., Jonas R. H., Scott M. L. Oxidative deterioration of the muscle proteins during nutritional muscular dystrophy in chicks. J Nutr. 1977 Oct;107(10):1786–1791. doi: 10.1093/jn/107.10.1786. [DOI] [PubMed] [Google Scholar]