Abstract
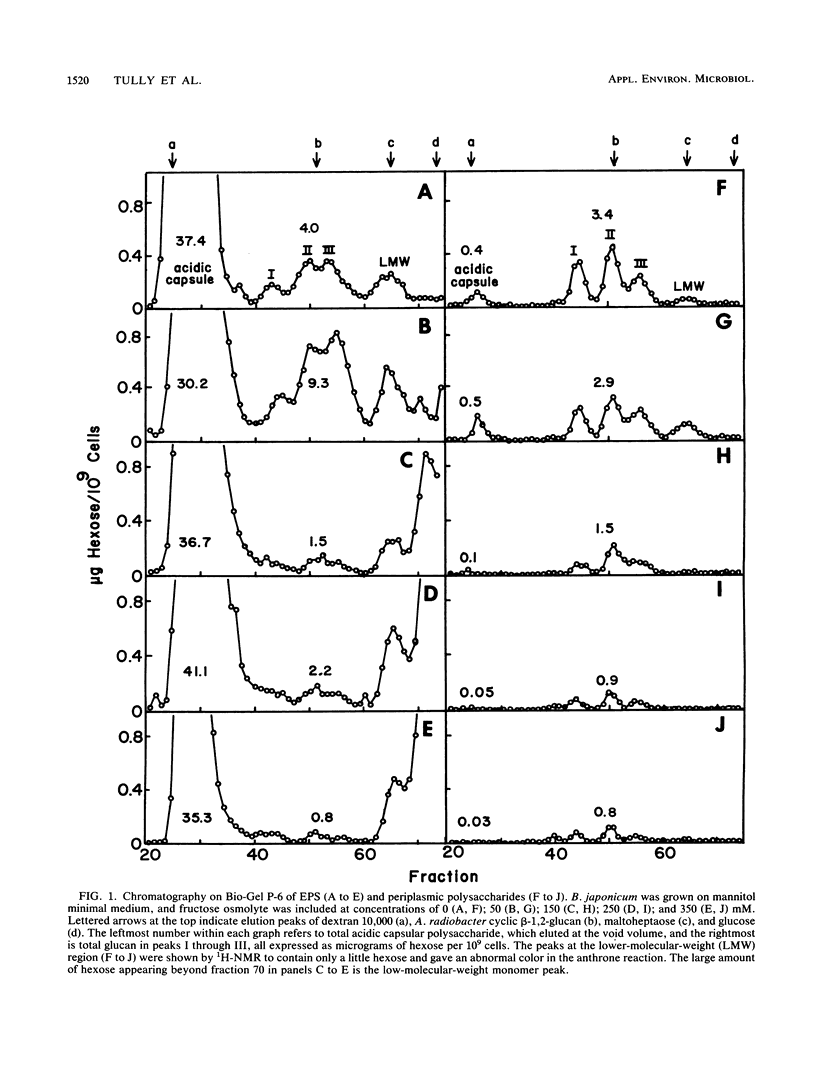
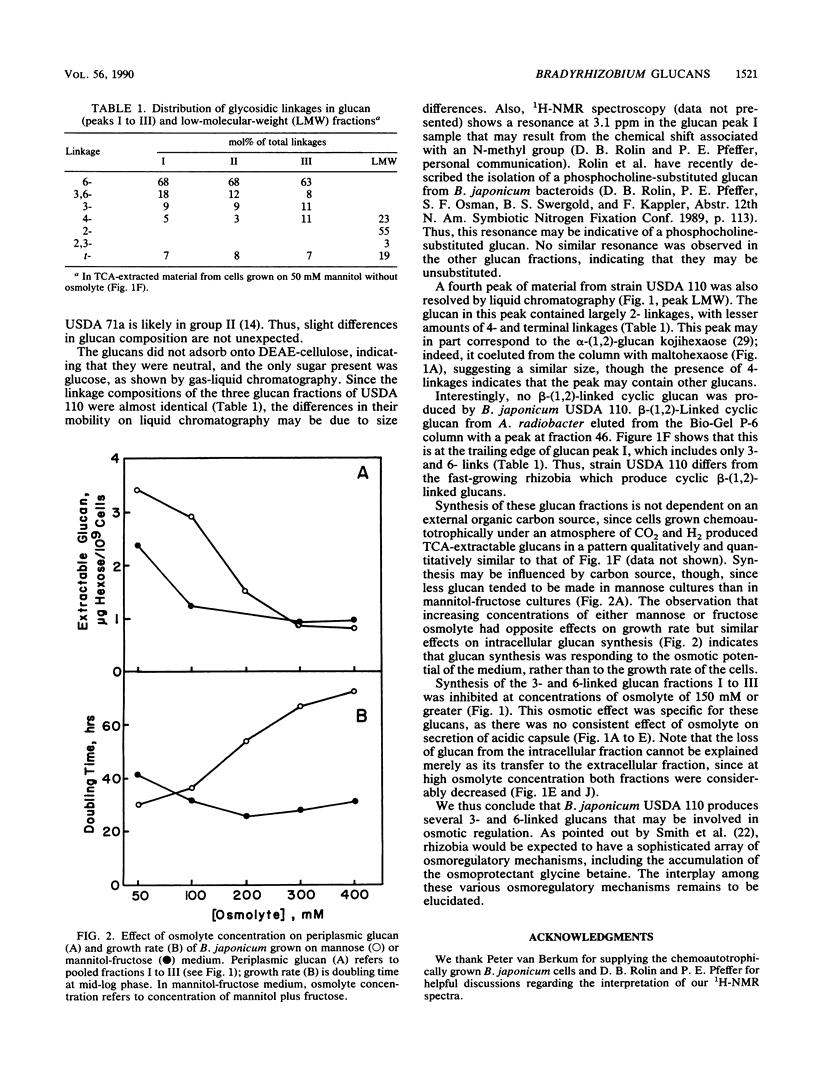
Bradyrhizobium japonicum USDA 110 synthesized both extracellular and periplasmic polysaccharides when grown on mannitol minimal medium. The extracellular polysaccharides were separated into a high-molecular-weight acidic capsular extracellular polysaccharide fraction (90% of total hexose) and three lower-molecular-weight glucan fractions by liquid chromatography. Periplasmic glucans, extracted from washed cells with 1% trichloroacetic acid, gave a similar pattern on liquid chromatography. Linkage analysis of the major periplasmic glucan fractions demonstrated mainly 6-linked glucose (63 to 68%), along with some 3,6- (8 to 18%), 3- (9 to 11%), and terminal (7 to 8%) linkages. The glucose residues were β-linked as shown by 1H-nuclear magnetic resonance analysis. Glucan synthesis by B. japonicum cells grown on mannitol medium with 0 to 350 mM fructose as osmolyte was measured. Fructose at 150 mM or higher inhibited synthesis of periplasmic and extracellular 3- and 6-linked glucans but had no effect on the synthesis of capsular acidic extracellular polysaccharides.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clark D. P. Mutant of Escherichia coli deficient in osmoregulation of periplasmic oligosaccharide synthesis. J Bacteriol. 1985 Mar;161(3):1049–1053. doi: 10.1128/jb.161.3.1049-1053.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HAKOMORI S. A RAPID PERMETHYLATION OF GLYCOLIPID, AND POLYSACCHARIDE CATALYZED BY METHYLSULFINYL CARBANION IN DIMETHYL SULFOXIDE. J Biochem. 1964 Feb;55:205–208. [PubMed] [Google Scholar]
- Huber T. A., Agarwal A. K., Keister D. L. Extracellular polysaccharide composition, ex planta nitrogenase activity, and DNA homology in Rhizobium japonicum. J Bacteriol. 1984 Jun;158(3):1168–1171. doi: 10.1128/jb.158.3.1168-1171.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P. Osmotic regulation and the biosynthesis of membrane-derived oligosaccharides in Escherichia coli. Proc Natl Acad Sci U S A. 1982 Feb;79(4):1092–1095. doi: 10.1073/pnas.79.4.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy E. P., Rumley M. K., Schulman H., Van Golde L. M. Identification of sn-glycero-1-phosphate and phosphoethanolamine residues linked to the membrane-derived Oligosaccharides of Escherichia coli. J Biol Chem. 1976 Jul 25;251(14):4208–4213. [PubMed] [Google Scholar]
- Miller K. J., Gore R. S., Johnson R., Benesi A. J., Reinhold V. N. Cell-associated oligosaccharides of Bradyrhizobium spp. J Bacteriol. 1990 Jan;172(1):136–142. doi: 10.1128/jb.172.1.136-142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller K. J., Kennedy E. P., Reinhold V. N. Osmotic adaptation by gram-negative bacteria: possible role for periplasmic oligosaccharides. Science. 1986 Jan 3;231(4733):48–51. doi: 10.1126/science.3941890. [DOI] [PubMed] [Google Scholar]
- Mort A. J., Bauer W. D. Composition of the Capsular and Extracellular Polysaccharides of Rhizobium japonicum: CHANGES WITH CULTURE AGE AND CORRELATIONS WITH BINDING OF SOYBEAN SEED LECTIN TO THE BACTERIA . Plant Physiol. 1980 Jul;66(1):158–163. doi: 10.1104/pp.66.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider J. E., Reinhold V., Rumley M. K., Kennedy E. P. Structural studies of the membrane-derived oligosaccharides of Escherichia coli. J Biol Chem. 1979 Oct 25;254(20):10135–10138. [PubMed] [Google Scholar]
- Schulman H., Kennedy E. P. Localization of membrane-derived oligosaccharides in the outer envelope of Escherichia coli and their occurrence in other Gram-negative bacteria. J Bacteriol. 1979 Jan;137(1):686–688. doi: 10.1128/jb.137.1.686-688.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith L. T., Pocard J. A., Bernard T., Le Rudulier D. Osmotic control of glycine betaine biosynthesis and degradation in Rhizobium meliloti. J Bacteriol. 1988 Jul;170(7):3142–3149. doi: 10.1128/jb.170.7.3142-3149.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., HARRISON J. S. Studies on yeast metabolism. I. Fractionation and microdetermination of cell carbohydrates. Biochem J. 1952 Jan;50(3):298–303. doi: 10.1042/bj0500298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E. Synthesis of Exopolysaccharide by Bradyrhizobium japonicum during Growth on Hydroaromatic Substrates. Appl Environ Microbiol. 1988 Jun;54(6):1624–1626. doi: 10.1128/aem.54.6.1624-1626.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tully R. E., Terry M. E. Decreased Exopolysaccharide Synthesis by Anaerobic and Symbiotic Cells of Bradyrhizobium japonicum. Plant Physiol. 1985 Oct;79(2):445–450. doi: 10.1104/pp.79.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zevenhuizen L. P., Scholten-Koerselman H. J. Surface carbohydrates of Rhizobium. I. Beta-1, 2-glucans. Antonie Van Leeuwenhoek. 1979;45(2):165–175. doi: 10.1007/BF00418581. [DOI] [PubMed] [Google Scholar]



