Abstract
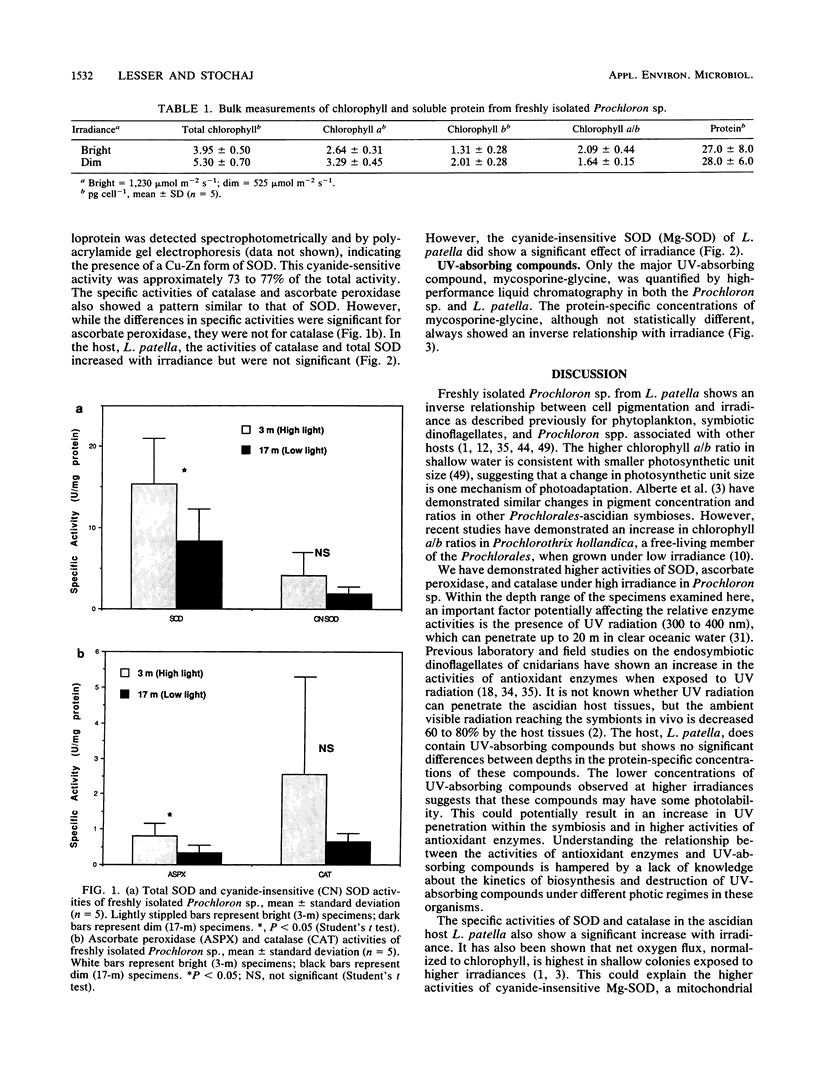
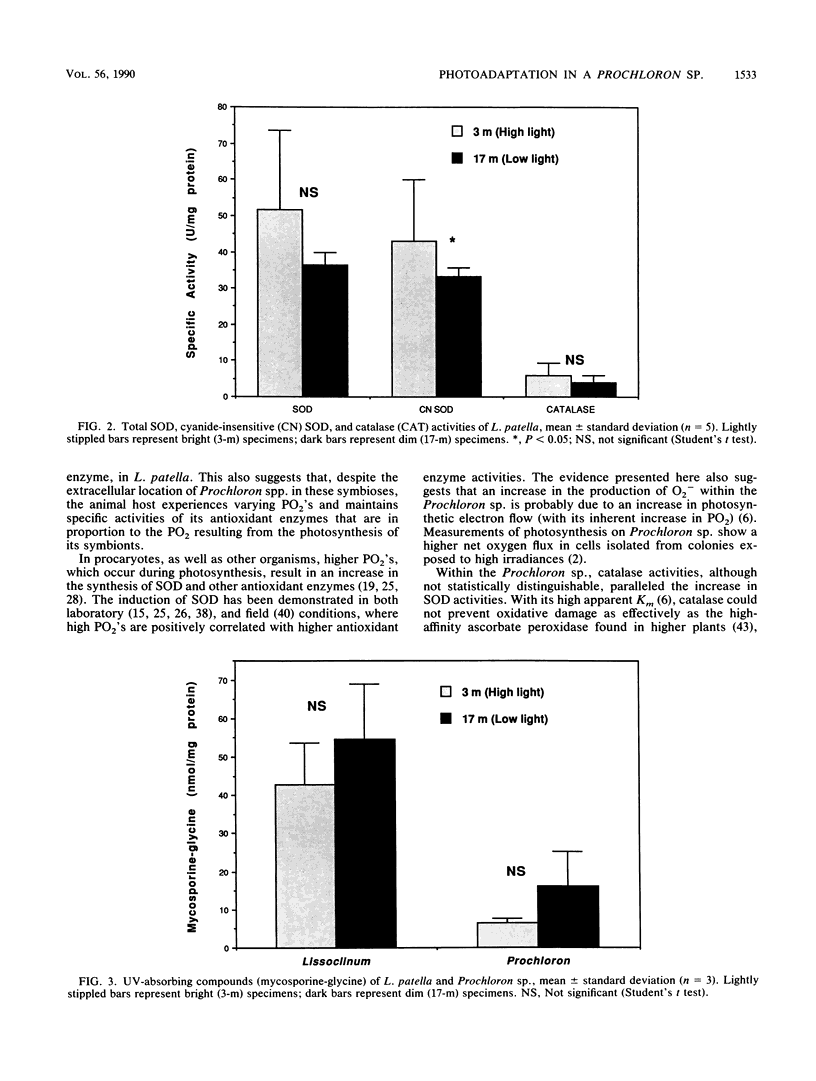
Superoxide dismutase, ascorbate peroxidase, and catalase activities were studied in the symbiotic photosynthetic procaryote Prochloron sp. and its ascidian host Lissoclinum patella. The protein-specific activities of these antioxidant enzymes in the Prochloron sp. and L. patella collected at different depths from the Great Barrier Reef, Australia, were directly proportional to irradiance, whereas the pigment concentrations in the Prochloron sp. were inversely proportional to irradiance. The presence of a cyanide-sensitive superoxide dismutase, presumably a Cu-Zn metalloprotein, in the Prochloron sp. extends the possible phylogenetic distribution of this protein. The concentration of UV-absorbing mycosporine-like amino acids is inversely proportional to irradiance in both the host and symbiont, suggesting that these compounds may not provide sufficient protection against UV radiation in high-irradiance environments. The significant differences in the specific activities of these antioxidant enzymes, cellular photosynthetic pigment concentrations, and UV-absorbing compounds from high- and low-irradiance habitats constitute an adaptive response to different photic environments. These photoadaptive responses are essential to prevent inhibition of photosynthesis by high fluxes of visible and UV radiation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEERS R. F., Jr, SIZER I. W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952 Mar;195(1):133–140. [PubMed] [Google Scholar]
- Bannister J. V., Bannister W. H., Rotilio G. Aspects of the structure, function, and applications of superoxide dismutase. CRC Crit Rev Biochem. 1987;22(2):111–180. doi: 10.3109/10409238709083738. [DOI] [PubMed] [Google Scholar]
- Burger-Wiersma T., Post A. F. Functional Analysis of the Photosynthetic Apparatus of Prochlorothrix hollandica (Prochlorales), a Chlorophyll b Containing Procaryote. Plant Physiol. 1989 Oct;91(2):770–774. doi: 10.1104/pp.91.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110. doi: 10.1146/annurev.bi.58.070189.000455. [DOI] [PubMed] [Google Scholar]
- Elstner E. F., Heupel A. Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem. 1976 Feb;70(2):616–620. doi: 10.1016/0003-2697(76)90488-7. [DOI] [PubMed] [Google Scholar]
- Fridovich I. Biological effects of the superoxide radical. Arch Biochem Biophys. 1986 May 15;247(1):1–11. doi: 10.1016/0003-9861(86)90526-6. [DOI] [PubMed] [Google Scholar]
- Fridovich I. The biology of oxygen radicals. Science. 1978 Sep 8;201(4359):875–880. doi: 10.1126/science.210504. [DOI] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Induction of superoxide dismutase by molecular oxygen. J Bacteriol. 1973 May;114(2):543–548. doi: 10.1128/jb.114.2.543-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory E. M., Fridovich I. Oxygen toxicity and the superoxide dismutase. J Bacteriol. 1973 Jun;114(3):1193–1197. doi: 10.1128/jb.114.3.1193-1197.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JERLOV N. G. Ultra-violet radiation in the sea. Nature. 1950 Jul 15;166(4211):111–112. doi: 10.1038/166111a0. [DOI] [PubMed] [Google Scholar]
- Jamieson D., Chance B., Cadenas E., Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- Lesser M. P. Photobiology of natural populations of zooxanthellae from the sea anemone Aiptasia pallida: assessment of the host's role in protection against ultraviolet radiation. Cytometry. 1989 Sep;10(5):653–658. doi: 10.1002/cyto.990100522. [DOI] [PubMed] [Google Scholar]
- Lewin R. A. Prochloron--a status report. Phycologia. 1984;23(2):203–208. doi: 10.2216/i0031-8884-23-2-203.1. [DOI] [PubMed] [Google Scholar]
- Mikell A. T., Parker B. C., Gregory E. M. Factors affecting high-oxygen survival of heterotrophic microorganisms from an antarctic lake. Appl Environ Microbiol. 1986 Dec;52(6):1236–1241. doi: 10.1128/aem.52.6.1236-1241.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morden C. W., Golden S. S. psbA genes indicate common ancestry of prochlorophytes and chloroplasts. Nature. 1989 Jan 26;337(6205):382–385. doi: 10.1038/337382a0. [DOI] [PubMed] [Google Scholar]
- Oyanagui Y. Reevaluation of assay methods and establishment of kit for superoxide dismutase activity. Anal Biochem. 1984 Nov 1;142(2):290–296. doi: 10.1016/0003-2697(84)90467-6. [DOI] [PubMed] [Google Scholar]
- Tel-Or E., Huflejt M. E., Packer L. Hydroperoxide metabolism in cyanobacteria. Arch Biochem Biophys. 1986 Apr;246(1):396–402. doi: 10.1016/0003-9861(86)90485-6. [DOI] [PubMed] [Google Scholar]
- Turner S., Burger-Wiersma T., Giovannoni S. J., Mur L. R., Pace N. R. The relationship of a prochlorophyte Prochlorothrix hollandica to green chloroplasts. Nature. 1989 Jan 26;337(6205):380–382. doi: 10.1038/337380a0. [DOI] [PubMed] [Google Scholar]
- Withers N. W., Alberte R. S., Lewin R. A., Thornber J. P., Britton G., Goodwin T. W. Photosynthetic unit size, carotenoids, and chlorophyll-protein composition of Prochloron sp., a prokaryotic green alga. Proc Natl Acad Sci U S A. 1978 May;75(5):2301–2305. doi: 10.1073/pnas.75.5.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]



