Abstract
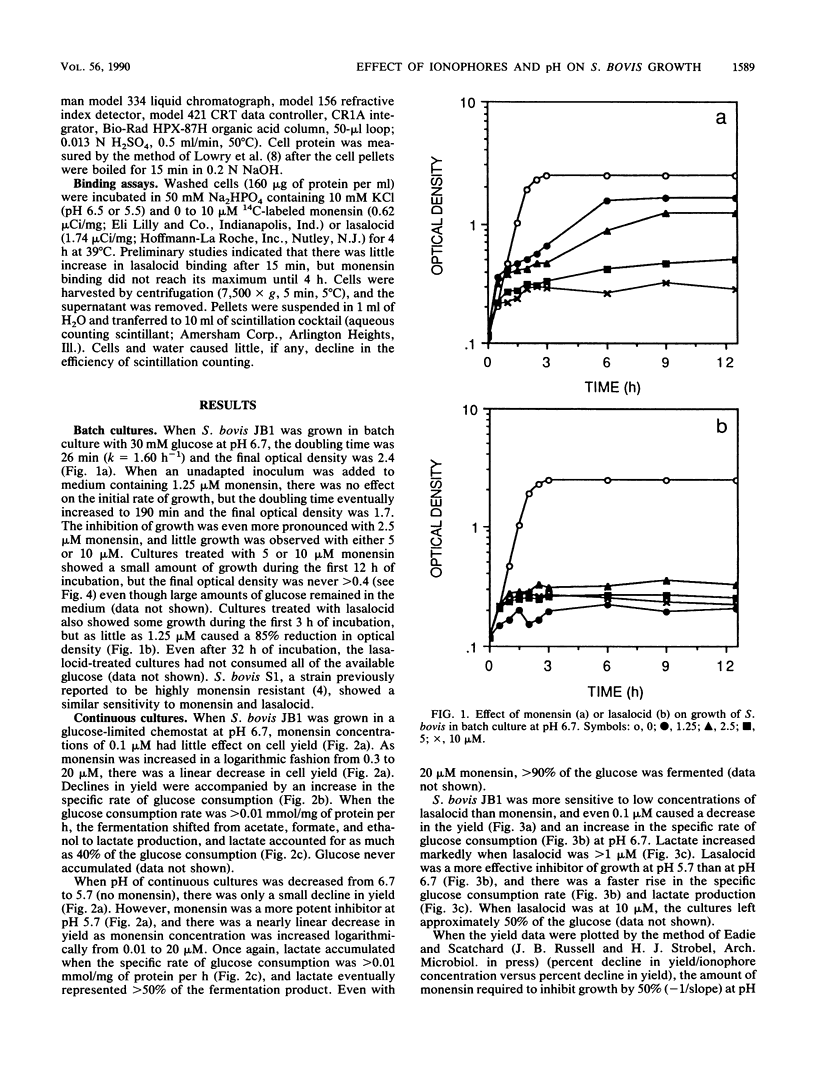
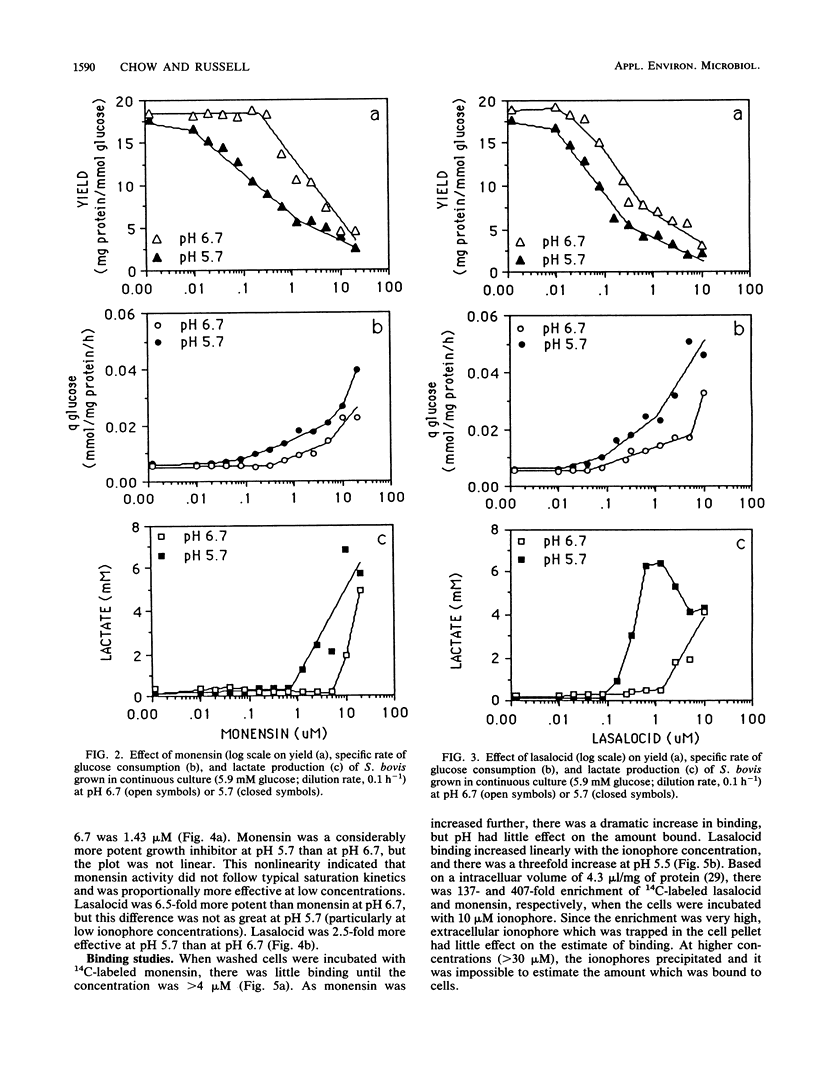
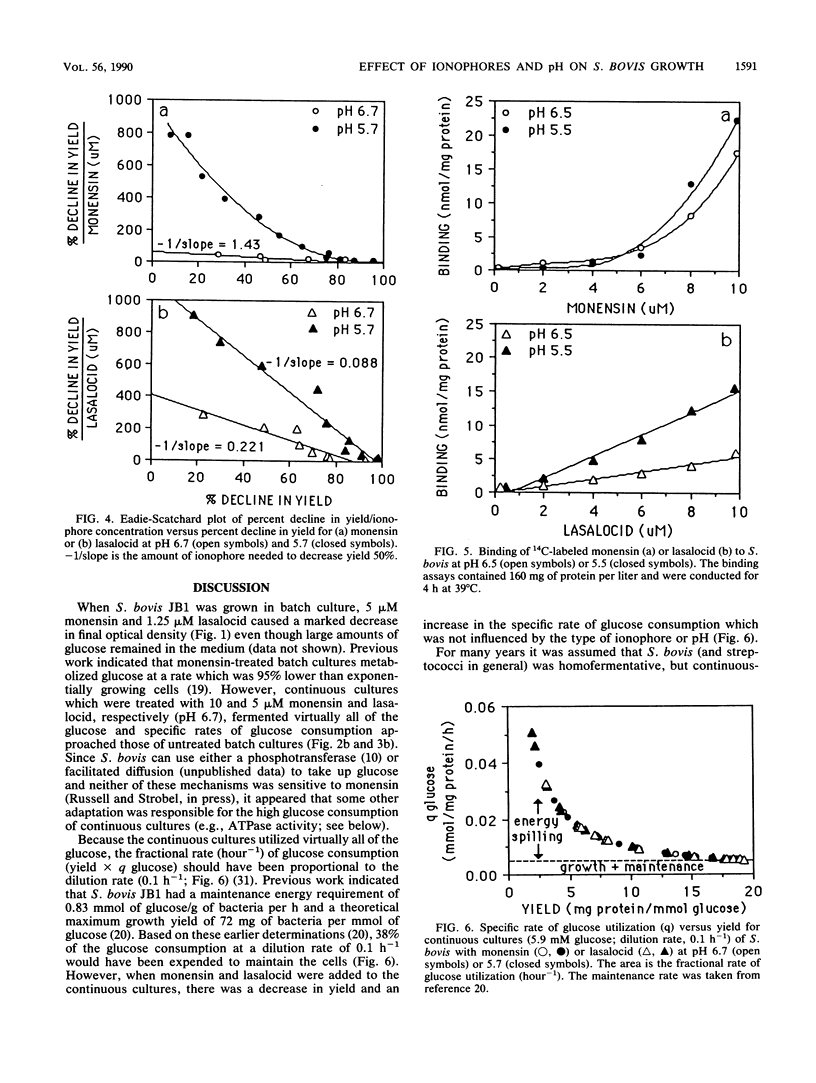
Batch cultures (pH 6.7) of Streptococcus bovis JB1 were severely inhibited by 1.25 and 5 microM lasalocid and monensin, respectively, even though large amounts of glucose remained in the medium. However, continuous cultures tolerated as much as 10 and 20 microM, respectively, and used virtually all of the glucose. Although continuous cultures grew with high concentrations of ionophore, the yield of bacterial protein decreased approximately 10-fold. When pH was decreased from 6.7 to 5.7, the potency of both ionophores increased, but lasalocid always caused a larger decrease in yield. The increased activity of lasalocid at pH 5.7 could largely be explained by an increased binding of the ionophore to the cell membrane. Because monensin did not show an increased binding at low pH, some other factor (e.g., ion turnover) must have been influencing its activity. There was a linear increase in lasalocid binding as the concentration increased, but monensin binding increased markedly at high concentrations. Based on the observations that (i) S. bovis cells bound significant amounts of ionophore (the ratio of ionophore to cell material was more important than the absolute concentration), (ii) batch cultures responded differently from continuous cultures, and (iii) pH can have a marked effect on ionophore activity, it appears that the term "minimum inhibitory concentration" may not provide an accurate assessment of microbial growth inhibition in vivo.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen M., Wolin M. J. Effect of monensin and lasalocid-sodium on the growth of methanogenic and rumen saccharolytic bacteria. Appl Environ Microbiol. 1979 Jul;38(1):72–77. doi: 10.1128/aem.38.1.72-77.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson K. A., Boling J. A. Effects of potassium ion concentrations on the antimicrobial activities of ionophores against ruminal anaerobes. Appl Environ Microbiol. 1987 Oct;53(10):2363–2367. doi: 10.1128/aem.53.10.2363-2367.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis S. M., Nagaraja T. G., Bartley E. E. Effects of lasalocid or monensin on lactate-producing or -using rumen bacteria. J Anim Sci. 1981 Feb;52(2):418–426. doi: 10.2527/jas1981.522418x. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E., DOUGHERTY R. W., BRYANT M. P., CELLO R. M. Microbiological and physiological changes associated with acute indigestion in sheep. Cornell Vet. 1952 Oct;42(4):423–449. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Martin S. A., Russell J. B. Transport and phosphorylation of disaccharides by the ruminal bacterium Streptococcus bovis. Appl Environ Microbiol. 1987 Oct;53(10):2388–2393. doi: 10.1128/aem.53.10.2388-2393.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir L. A., Barreto A., Jr Sensitivity of Streptococcus bovis to various antibiotics. J Anim Sci. 1979 Mar;48(3):468–473. doi: 10.2527/jas1979.483468x. [DOI] [PubMed] [Google Scholar]
- Nagaraja T. G., Taylor M. B. Susceptibility and resistance of ruminal bacteria to antimicrobial feed additives. Appl Environ Microbiol. 1987 Jul;53(7):1620–1625. doi: 10.1128/aem.53.7.1620-1625.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold C. J., Wallace R. J. Effects of the ionophores monensin and tetronasin on simulated development of ruminal lactic acidosis in vitro. Appl Environ Microbiol. 1988 Dec;54(12):2981–2985. doi: 10.1128/aem.54.12.2981-2985.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirt S. J. Maintenance energy: a general model for energy-limited and energy-sufficient growth. Arch Microbiol. 1982 Dec 3;133(4):300–302. doi: 10.1007/BF00521294. [DOI] [PubMed] [Google Scholar]
- Pirt S. J. The maintenance energy of bacteria in growing cultures. Proc R Soc Lond B Biol Sci. 1965 Oct 12;163(991):224–231. doi: 10.1098/rspb.1965.0069. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Biological applications of ionophores. Annu Rev Biochem. 1976;45:501–530. doi: 10.1146/annurev.bi.45.070176.002441. [DOI] [PubMed] [Google Scholar]
- Pressman B. C. Properties of ionophores with broad range cation selectivity. Fed Proc. 1973 Jun;32(6):1698–1703. [PubMed] [Google Scholar]
- Russell J. B. A proposed mechanism of monensin action in inhibiting ruminal bacterial growth: effects on ion flux and protonmotive force. J Anim Sci. 1987 May;64(5):1519–1525. doi: 10.2527/jas1987.6451519x. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Baldwin R. L. Comparison of maintenance energy expenditures and growth yields among several rumen bacteria grown on continuous culture. Appl Environ Microbiol. 1979 Mar;37(3):537–543. doi: 10.1128/aem.37.3.537-543.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Robinson P. H. Compositions and characteristics of strains of Streptococcus bovis. J Dairy Sci. 1984 Jul;67(7):1525–1531. doi: 10.3168/jds.S0022-0302(84)81471-X. [DOI] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J. Effect of ionophores on ruminal fermentation. Appl Environ Microbiol. 1989 Jan;55(1):1–6. doi: 10.1128/aem.55.1.1-6.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell J. B., Strobel H. J. Effects of additives on in vitro ruminal fermentation: a comparison of monensin and bacitracin, another gram-positive antibiotic. J Anim Sci. 1988 Feb;66(2):552–558. doi: 10.2527/jas1988.662552x. [DOI] [PubMed] [Google Scholar]
- Sandeaux R., Seta P., Jeminet G., Alleaume M., Gavach C. The influence of pH on the conductance of lipid bimolecular membranes in relation to the alkaline ion transport induced by carboxylic carriers grisorixin, alborixin and monensin. Biochim Biophys Acta. 1978 Aug 17;511(3):499–508. doi: 10.1016/0005-2736(78)90284-5. [DOI] [PubMed] [Google Scholar]
- Slyter L. L. Influence of acidosis on rumen function. J Anim Sci. 1976 Oct;43(4):910–929. doi: 10.2527/jas1976.434910x. [DOI] [PubMed] [Google Scholar]
- Strobel H. J., Russell J. B. Non-proton-motive-force-dependent sodium efflux from the ruminal bacterium Streptococcus bovis: bound versus free pools. Appl Environ Microbiol. 1989 Oct;55(10):2664–2668. doi: 10.1128/aem.55.10.2664-2668.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J. FRUCTOSE-1,6-DIPHOSPHATE REQUIREMENT OF STREPTOCOCCAL LACTIC DEHYDROGENASES. Science. 1964 Nov 6;146(3645):775–777. doi: 10.1126/science.146.3645.775. [DOI] [PubMed] [Google Scholar]



