Abstract
Cardiac reperfusion and aging are associated with increased rates of mitochondrial free radical production. Mitochondria are therefore a likely site of reperfusion-induced oxidative damage, the severity of which may increase with age. 4-Hydroxy-2-nonenal (HNE), a major product of lipid peroxidation, increases in concentration upon reperfusion of ischemic cardiac tissue, can react with and inactivate enzymes, and inhibits mitochondrial respiration in vitro. HNE modification of mitochondrial protein(s) might, therefore, be expected to occur during reperfusion and result in loss in mitochondrial function. In addition, this process may be more prevalent in aged animals. To begin to test this hypothesis, hearts from 8- and 24-month-old rats were perfused in Langendorff fashion and subjected to periods of ischemia and/or reperfusion. The rate of state 3 respiration of mitochondria isolated from hearts exposed to ischemia (25 min) was approximately 25% less than that of controls, independent of age. Reperfusion (40 min) caused a further decline in the rate of state 3 respiration in hearts isolated from 24- but not 8-month-old rats. Furthermore, HNE modification of mitochondrial protein (∼30 and 44 kDa) occurred only during reperfusion of hearts from 24-month-old rats. Thus, HNE-modified protein was present in only those mitochondria exhibiting reperfusion-induced declines in function. These studies therefore identify mitochondria as a subcellular target of reperfusion damage and a site of age-related increases in susceptibility to injury.
Oxygen free radicals increase in concentration upon reperfusion of ischemic cardiac tissue (1–11). It is well established that these reactive species can interact with and damage various cellular components (for review, see refs. 12–14). Thus, although restoration of blood flow is the sole method for salvaging ischemic tissue, oxidative damage may occur during reoxygenation and contribute to ischemia–reperfusion injury. Free radical production in the heart has also been reported to increase with age (15–19). Myocardium of senescent animals may, therefore, be more susceptible to reperfusion-induced oxidative damage. Cellular and subcellular targets of free radical damage and the mechanism and contribution of these events to myocardial ischemia–reperfusion injury have not, however, been established.
Free radicals, such as hydroxyl radical, are highly reactive and short-lived species. They would therefore be expected to cause damage at or near the site of their formation. The mitochondrial respiratory chain represents a major subcellular source of free radical species during reperfusion of ischemic myocardium (20, 21). In addition, myocardial ischemia–reperfusion has been associated with decreased rates of NADH-linked state 3 (ADP-dependent) respiration (22–27). Of particular importance to this study, heart mitochondria isolated from senescent animals exhibit higher rates of oxygen radical production than those from young animals (15–19). Thus, mitochondria are a likely site of reperfusion-induced oxidative damage, the severity of which may increase with age. It is therefore critical to determine whether cardiac reperfusion results in free radical damage to mitochondria and the mechanisms by which free radicals mediate declines in respiratory function.
Polyunsaturated fatty acids of membrane lipids are highly susceptible to peroxidation by oxygen radicals (12). Not surprisingly, reperfusion-induced increases in the level of free radicals are paralleled by elevated rates of lipid peroxidation (28–34). Peroxidation of membrane lipids results in the fragmentation of polyunsaturated fatty acids giving rise to various aldehydes, alkenals, and hydroxyalkenals such as malonaldehyde and 4-hydroxy-2-nonenal (HNE) (12). Many of these products are cytotoxic when introduced into cells in culture and into whole animals, effects believed to be mediated in large part by their reactivity toward protein (12). HNE, a major product of lipid peroxidation, is thought to be the most reactive of these compounds and is, therefore, an important mediator of free radical damage (12). HNE reacts with protein at the sulfhydryl group of cysteine, the imidazole nitrogen(s) of histidine, and/or the ɛ-amine of lysine (12, 35–38), resulting in enzyme inactivation (12, 39–41). In addition, the concentration of HNE in the perfusate has been reported to increase upon reperfusion of ischemic myocardium (32), and we have recently demonstrated that treatment of intact cardiac mitochondria with HNE results in declines in NADH-linked respiration (42). Mitochondria, a source of free radicals during reperfusion (20, 21), are therefore likely targets of lipid peroxidation and HNE-mediated dysfunction.
We propose that reperfusion-induced mitochondrial dysfunction increases in an age-dependent manner and is mediated, in part, by modification of specific mitochondrial protein(s) by the lipid peroxidation product HNE. To begin to test this hypothesis, we sought evidence for age-related differences in reperfusion-induced mitochondrial dysfunction and HNE modification to mitochondrial protein. Hearts isolated from adult (8 month old) and senescent (24 month old) rats were subjected to perfusion, ischemia, and reperfusion. Mitochondria were then isolated and respiratory activities were evaluated. In addition, the level and relative distribution of HNE-modified protein were assessed. We present data that demonstrates that the degree of mitochondrial dysfunction associated with reperfusion is dramatically increased with age. In parallel with this finding, we report that HNE modification of mitochondrial protein occurs during reperfusion exclusively in hearts isolated from senescent rats. These studies lend support for our hypothesis and provide the basis for future experiments aimed at establishing a direct link among free radical damage, mitochondrial dysfunction, aging, and cardiac reperfusion injury.
MATERIALS AND METHODS
Preparation and Perfusion of Isolated Rat Heart.
After intraperitoneal injection of heparin (500 units) and administration of sodium pentobarbitol, hearts were isolated from 8- and 24-month-old male Fisher 344 rats (National Institute of Aging colony). Hearts were then placed in ice-cold modified Krebs–Henseleit buffer (120 mM NaCl/4.8 mM KCl/2.0 mM CaCl2/1.25 mM MgCl2/1.25 mM KH2PO4/25 mM NaHCO3/10 mM glucose/5 units of insulin per liter) to halt metabolic activities and minimize any ischemic damage arising from the isolation protocol. Extraneous tissue was rapidly removed, the aorta was cannulated, and the heart was perfused in retrograde fashion according to Langendorff (43) with modified Krebs–Henseleit buffer, at 37°C, saturated with 95% O2/5% CO2. Hearts were placed in a water-jacketed chamber (37°C) and perfusion pressure was maintained at 60 mmHg (1 mmHg = 133 Pa). A Millar pressure transducer within a latex balloon was placed into the left ventricle and the balloon was inflated to an initial left ventricular end diastolic pressure of 5 mmHg. Hearts were paced at 300 beats per min to remove the variable of heart rate and accurately measure left ventricular pressure and dP/dt. Elapsed time between isolation of the heart and perfusion was approximately 2 min. Experiments consisted of the following protocols: (i) a 90-min normoxic perfusion, (ii) a 25-min perfusion followed by a 25-min no-flow global ischemia, or (iii) a 25-min perfusion followed by a 25-min ischemia and then a 40-min reperfusion. During the equilibration period, basal hemodynamic parameters were established.
Isolation of Mitochondria.
Subsarcolemmal mitochondria were isolated from ventricles as follows: Ventricles were rapidly removed, then immersed, and rinsed in ice-cold buffer containing 180 mM KCl, 5.0 mM Mops, and 2.0 mM EGTA at pH 7.25 (buffer A). The ventricles were blotted dry, weighed (0.8–1.0 g), and then minced with scissors followed by homogenization in 20 ml of buffer A per g of ventricles with a Polytron homogenizer (low setting, 3 s). The homogenate was then centrifuged at 500 × g for 7.5 min at 4°C. The supernatant was filtered through cheese cloth and centrifuged at 5,000 × g for 10 min at 4°C. The resulting mitochondrial pellet was washed two times and resuspended into 150 μl of buffer A to a final protein concentration of approximately 25 mg/ml. Protein determinations were made by using the BCA method (Pierce), with BSA as a standard. Mitochondria were kept at 4°C prior to various analyses and exhibited no change in state 3 or state 4 respiratory rates for up to 3.0 h.
Evaluation of Mitochondrial O2 Consumption.
ADP-independent (state 4) and -dependent (state 3) respiration were measured by using a Clark-type oxygen electrode (Instech, Plymouth Meeting, PA). Mitochondria were diluted to a protein concentration of 0.5 mg/ml in assay buffer (120 mM KCl/5.0 mM KH2PO4/5.0 mM Mops/1.0 mM EGTA at pH 7.25). State 2 respiration was initiated by the addition of glutamate (15 mM). After 2.0 min, state 3 respiration was initiated by addition of ADP (0.5 mM). Upon depletion of ADP, state 4 respiration was monitored. For uncoupled respiration, 60 μM 2,4-dinitrophenol was added instead of ADP. The substrate concentrations and assay buffer used were optimized to yield maximal rates of respiration.
Detection of HNE-Modified Mitochondrial Protein.
Western blot analysis was used to assess the molecular weight distribution and selectivity of HNE modifications to protein. It has previously been shown that the sulfhydryl group of cysteine, the imidazole nitrogen(s) of histidine, and the ɛ-amine of lysine readily react with the double bond (C3) of HNE to form the respective Michael adducts (12, 35–38). The polyclonal antibody preparation used in this study was prepared as described (44) and is highly specific to HNE-derived modifications to protein, exhibiting no binding to malonaldehyde-protein adducts or to Michael adducts formed between Nα-acetylcysteine and acrolein, t-2-pentenal, or t-2-nonenal. Antibody binding requires the presence of the 4-hydroxyl group, is sensitive to the chain length of the modifying 4-hydroxy-2-alkenal, and, as judged by competitive ELISA experiments, recognizes cysteine-, histidine-, and lysine-HNE Michael adducts (44, 45). For Western blot analysis, samples were prepared by addition of mitochondrial solution (1.0 mg of protein per ml of 10 mM K2HPO4/100 mM NaCl/1.0 mM EGTA, pH 7.25) to an equal volume of 2× gel sample buffer (126 mM Tris⋅HCl/20% glycerol/4% SDS/1.0% 2-mercaptoethanol/0.005% bromophenol blue at pH 6.8). Samples were then loaded onto duplicate 10% Tris/glycine polyacrylamide gels (12.5 μg of protein per lane) and component proteins were resolved by electrophoresis (Novex Minicell II). One gel of a series was stained with Coomassie blue to assess protein profiles. Protein contained in the second gel was electroblotted onto a nitrocellulose membrane and HNE-modified protein was visualized by using an alkaline-phosphatase based chemiluminscence assay (reagents and protocol from Tropix, Bedford, MA). Briefly, binding of primary antibody (1:8,000 dilution in blocking buffer) was detected by using goat anti-rabbit IgG conjugated to alkaline phosphatase. After incubation with a chemilumniscent alkaline phosphatase substrate, primary antibody bound to HNE-modified proteins was observed by autoradiography.
RESULTS
Effects of Ischemia and Reperfusion on Mitochondrial Respiratory Activities.
Mitochondrial dysfunction induced by reperfusion was found to be dependent on age. Hearts from adult (8 month old) and senescent (24 month old) rats were exposed to perfusion, ischemia, or reperfusion. Mitochondria isolated from perfused hearts of both age groups exhibited similar rates of NADH-linked ADP-dependent (state 3) respiration (Fig. 1). State 3 respiratory rates were 191.7 ± 18.3 and 191.9 ± 12.6 nmol of O per min per mg of protein for cardiac mitochondria from 8- and 24-month-old rats, respectively (Table 1). Respiratory rates of mitochondria isolated from hearts immediately after euthanasia were nearly identical (results not shown), indicating that 90 min of normoxic perfusion had no effect on baseline activities. Exposure of hearts from 8- and 24-month-old rats to 25 min of ischemia resulted in a 25% and 22% loss in the rate of state 3 respiration, respectively. No further decline was observed when hearts from 8-month-old rats were exposed to 40 min of reperfusion. In contrast, reperfusion of hearts from 24-month-old rats resulted in a 29% loss in mitochondrial state 3 respiratory activity relative to ischemic values. Observed declines in respiratory rates were not likely due to events occurring during or after isolation because mitochondrial activities remained stable for 3 h. These results demonstrate an age-related loss in mitochondrial function as a result of cardiac reperfusion.
Figure 1.
Age-related differences in the effect of ischemia and reperfusion on ADP-dependent mitochondrial respiration. After perfusion (P), ischemia (I), or reperfusion (R) of hearts from 8- and 24-month-old rats, mitochondria were isolated and respiratory activities were assessed by using a Clark-style oxygen electrode. Mitochondria at a concentration of 0.5 mg/ml were incubated with 15 mM glutamate for 2.0 min at 25°C. State 3 respiration was then initiated by addition of 0.5 mM ADP.
Table 1.
Mitochondrial yield and respiratory activity
| Protocol | Yield, mg/g of heart | State 3, nmol of O per min per mg | State 4, nmol of O per min per mg | RCR State 3/state 4 | ADP/O | Uncoupled, nmol of O per min per mg |
|---|---|---|---|---|---|---|
| 8 month old | ||||||
| Perfused (n = 8) | 8.8 ± 0.8 | 191.7 ± 18.3* | 26.6 ± 4.5 | 7.5 ± 1.3 | 3.1 ± 0.1 | 186.8 ± 24.3** |
| Ischemic (n = 6) | 8.6 ± 0.9 | 143.0 ± 17.9* | 28.6 ± 0.8 | 5.0 ± 0.7 | 3.2 ± 0.3 | 151.2 ± 25.7** |
| Reperfused (n = 7) | 8.4 ± 1.3 | 153.7 ± 20.8§ | 33.8 ± 4.4 | 4.6 ± 0.7 | 2.9 ± 0.2 | 149.0 ± 24.8§§ |
| 24 month old | ||||||
| Perfused (n = 5) | 8.6 ± 0.8 | 191.9 ± 12.6† | 30.2 ± 3.2 | 6.4 ± 0.4 | 2.9 ± 0.2 | 190.2 ± 12.5‡‡ |
| Ischemic (n = 5) | 8.2 ± 1.1 | 149.0 ± 15.4†‡ | 31.2 ± 4.2 | 4.8 ± 0.6 | 2.9 ± 0.1 | 156.7 ± 11.4‡‡†† |
| Reperfused (n = 8) | 8.8 ± 1.6 | 105.4 ± 8.9§‡ | 27.0 ± 3.9 | 4.0 ± 0.4 | 2.8 ± 0.2 | 94.7 ± 15.8§§†† |
Values are the mean ± SD. RCR, respiratory control ratio. P values (determined from paired t test) where like symbols indicate values compared. State 3: *, ≤0.001;
, ≤0.004;
, ≤0.003;
, ≤ 0.001. Uncoupled: **, ≤0.03;
, ≤0.008;
, ≤0.0001;
, ≤0.001. Comparison of state 3 values with those of uncoupled respiration at a given experimental protocol (i.e., 8-month-old perfused state 3 vs. perfused uncoupled) indicated that differences were not statistically significant.
Inclusion of compounds (2,4-dinitrophenol) that abolish the mitochondrial proton gradient results in maximum rates of electron transport and O2 consumption independent of ADP transport or ATPase activity. Therefore, if damage to adenine nucleotide translocase (ANT) or ATPase contribute to observed declines in ADP-dependent respiration (Fig. 1), uncoupled respiration would be affected to a lesser extent. Changes in the rate of uncoupled respiration as a function of ischemia, reperfusion, and age paralleled and were not statistically different from those observed for ADP-dependent respiration (Table 1). Thus, although inhibition of or damage to ANT or ATPase may occur, this does not appear to contribute significantly to observed declines in the rate of mitochondrial respiration. Furthermore, under the conditions of our experiments, ischemia–reperfusion does not bring about global disruption of the mitochondrial membrane. No significant changes in state 4 respiration, ADP/O, or mitochondrial yield were observed as a function of age, ischemia, or reperfusion (Table 1). Age-related reperfusion-induced declines in mitochondrial respiration, therefore, reflect damage to electron transport chain components and/or a reduction in the supply of reducing equivalents (NADH).
Hemodynamic Function.
Coronary flow (8.1 ± 0.3 ml/min), developed tension (139.5 ± 10.1 mmHg), and contractility (dP/dtmax = 14, 555 ± 870 mmHg/sec) remained stable in hearts throughout 90 min of normoxic perfusion, exhibiting no age-related differences. Reperfusion of ischemic heart resulted in a rapid recovery of hemodynamic functions to approximately 70% of the values observed prior to ischemia. Although mitochondrial respiration is depressed after reperfusion of hearts from 24-month-old rats, the degree of hemodynamic recovery was independent of age. During retrograde perfusion, hearts are required to perform minimal work. Therefore, hemodynamic parameters may not be valid indicators of mitochondrial viability under the conditions of our experiments. This model does, however, provide a convenient system for evaluating changes in mitochondrial function induced by different perfusion protocols. The duration of ischemia used in this study was chosen to minimize necrotic damage. This ensured that alterations in mitochondrial function could be attributed to events that occurred during reperfusion rather than a loss of perfusion to portions of the myocardium compromised by ischemia.
Modification of Mitochondrial Protein by HNE.
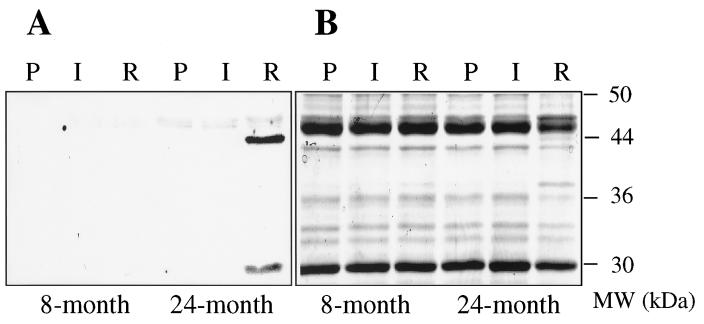
To obtain direct evidence for HNE-derived damage to mitochondrial protein as a function of reperfusion and age, cardiac mitochondria were isolated from rat hearts after perfusion, ischemia, or reperfusion and probed for the presence of HNE-modified protein by Western blot analysis. Polyclonal antibodies that recognize Michael adducts formed between HNE and cysteine, lysine, or histidine residues on protein were used in these studies (44, 45). As shown in Fig. 2A, no antibody binding was detected in mitochondria isolated from hearts of adult (8 month old) or senescent (24 month old) rats exposed to either perfusion or ischemia. Analysis of mitochondria from adult (8 month old) hearts subjected to reperfusion revealed no HNE-derived modifications to protein. In striking contrast, reperfusion of senescent (24 month old) hearts led to modification of two mitochondrial proteins by HNE. As judged by Western blot analysis, these proteins had approximate molecular masses of 30 and 44 kDa. Antibody binding to both proteins was competitively inhibited by amino acid-HNE adducts, thereby confirming that modification was due to HNE. The same pattern of antibody binding presented in Fig. 2A was observed in mitochondria isolated from each set of perfused, ischemic, and reperfused adult or senescent hearts. Without exception, HNE-modified mitochondrial protein was detected exclusively after reperfusion of senescent rat heart. Coomassie staining (Fig. 2B) revealed that the 44-kDa protein was present only in mitochondria from senescent rat heart exposed to reperfusion, suggesting that HNE modification alters electrophoretic mobility of the protein or this protein represents a proteolytic fragment. Experiments are currently underway to identify HNE-modified proteins and quantitate the level of modification.
Figure 2.
SDS/gel electrophoresis and Western blot analysis of protein from cardiac mitochondria. Hearts obtained from 8- and 24-month-old rats were exposed to perfusion (P), ischemia (I), or reperfusion (R). Mitochondria were then isolated and proteins were resolved by SDS/gel electrophoresis using duplicate 10% gels (12.5 μg of total protein per lane). One gel of the series was transferred to a nitrocellulose membrane for Western blot analysis with anti-HNE antibody (A). The second gel was stained with Coomassie blue for analysis of the molecular weight distribution of mitochondrial protein (B).
In keeping with our hypothesis, HNE-modified protein was observed (Fig. 2) in only those mitochondria exhibiting reperfusion-induced declines in function (Fig. 1). On the basis of Western blot analysis of pure protein with a known number of HNE-modified amino acids (39), the signal intensity of the 30- and 44-kDa bands shown in Fig. 2A represents approximately 0.3 and 1.2 pmol of HNE-modified amino acids, respectively. The antibody preparation and Western blot conditions used in this study allow for detection of as little as 0.1 pmol of amino acid-HNE adducts on a single protein (data not shown). Thus, although mitochondria isolated from adult rat hearts exposed to reperfusion may contain HNE-modified protein undetectable by the current assay (less than 0.1 pmol per single protein), it is clear that the level is much lower than that observed in mitochondria from reperfused senescent rat heart (Fig. 2). Rigorous quantification of the level of HNE modification requires protein isolation and direct chemical analysis.
DISCUSSION
Recent advances in capabilities for restoring flow to ischemic myocardial tissue, particularly through application of thrombolytic agents and improved surgical techniques, have heightened interest in potential deleterious events that occur during reperfusion. Because free radical production increases during cardiac reperfusion, oxidative damage is thought to result in myocardial injury upon reoxygenation (1–11). Cells, however, contain numerous antioxidant activities. Increases in free radical production are, therefore, not an accurate indication of oxidative damage. To clarify the role of free radicals, it is important to first determine whether free radical damage occurs at sites of reperfusion-induced dysfunction. Cardiac mitochondria, critical to the energy status and function of the heart, are a source of free radicals during reperfusion (20, 21) and exhibit increased rates of free radical production with age (15–19). In this study, we provide evidence that cardiac mitochondria exhibit an age-related increase in susceptibility to reperfusion-induced dysfunction. Reperfusion-induced declines in the rate of mitochondrial respiration reflect damage to electron transport components and/or a drop in the supply of reducing equivalents (NADH). Furthermore, HNE, an important mediator of free radical damage, was shown to modify mitochondrial protein. Modification appeared specific to certain proteins and occurred exclusively during reperfusion of hearts isolated from 24-month-old rats. Thus, HNE-modified protein was present in only those mitochondria exhibiting reperfusion-induced declines in function. These studies therefore identify mitochondria as a subcellular target of reperfusion damage and a site of age-related increases in susceptibility to injury.
In attempts to clarify the contribution of free radicals to specific metabolic and physiological processes altered by ischemia–reperfusion, experiments using a wide array of antioxidants have yielded conflicting results (for review, see ref. 46). This is likely due to the multifactorial nature of ischemia–reperfusion injury. Certain pathophysiological mechanisms may predominate depending on the conditions of the experiment. If significant cellular necrosis has occurred during the preceding ischemic event, antioxidant interventions during reperfusion may have little effect. Mitochondria irreversibly damaged by prolonged ischemia may not be targets for reperfusion-induced free radical damage due to a reduced ability to respire after reflow. In addition, depending on the model studied, the site of free radical generation (i.e., mitochondrial electron transport, xanthine oxidase activity, or neutrophils) may not be accessible to certain antioxidants. Finally, few studies have investigated age-related differences in the susceptibility to reperfusion-induced oxidative damage at the mitochondrial and/or molecular level. We have shown that modification of mitochondrial proteins by the lipid peroxidation product HNE occurs solely upon reperfusion of senescent heart. This observation suggests that aging may predispose cardiac mitochondria to reperfusion-induced oxidative damage, possibly due to a number of factors, including age-related (i) increases in the production of free radicals by mitochondria, (ii) decreases in the ability to protect against oxidative damage, and/or (iii) changes in mitochondrial composition and structure.
The results of this study lend support to the hypothesis that reperfusion-induced declines in mitochondrial respiration are due, in part, to modification of specific mitochondrial protein(s) by the lipid peroxidation product HNE and that these processes contribute to age-related increases in myocardial reperfusion injury. Future studies must identify those proteins modified by HNE and the effects of this modification on protein function. Mechanisms by which lipid peroxidation affects specific mitochondrial processes during reperfusion will be defined by establishing relationships between the level and identity of mitochondrial proteins inactivated by HNE and those exhibiting reperfusion-induced declines in activity. Studies addressing the effects of age, varying durations of ischemia and reperfusion, and cardiac work load will define conditions under which these mechanisms play a role. It is important to note that the studies described in this report were carried out on adult (8 month old) and senescent (24 month old) rats. Although we establish a clear difference in the level of reperfusion-induced mitochondrial dysfunction between these two ages, these studies do not indicate when during the process of aging susceptibility to reperfusion injury increases. Future work must address this question by using animals of intermediate ages. Furthermore, the relationship between aging and susceptibility to reperfusion injury is likely to be affected by the duration of ischemia and reperfusion. Clearly, identification of specific events contributing to myocardial reperfusion injury and conditions under which they occur is necessary for the design and implementation of interventions to diminish or prevent damage.
Acknowledgments
This work was supported by a Scientist Development Grant from the American Heart Association (9630025N) and with funds contributed, in part, by the American Heart Association, Northeast Ohio Affiliate. Partial support for this project was provided by a Case Western Reserve University Pepper Center Pilot Grant.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviation: HNE, 4-hydroxy-2-nonenal.
References
- 1.Garlick P B, Davies M J, Davies, Hearse D J, Slater T F. Circ Res. 1987;61:757–760. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- 2.Zweier J L, Flaherty J T, Weisfeldt M L. Proc Natl Acad Sci USA. 1987;84:1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bolli R, Patel B S, Jeroudi M O, Lai E K, McCay P B. J Clin Invest. 1988;82:476–485. doi: 10.1172/JCI113621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Flaherty J T, Weisfeldt M L. Free Radical Biol Med. 1988;5:409–419. doi: 10.1016/0891-5849(88)90115-3. [DOI] [PubMed] [Google Scholar]
- 5.McCord J M. Free Radical Biol Med. 1988;4:9–14. doi: 10.1016/0891-5849(88)90005-6. [DOI] [PubMed] [Google Scholar]
- 6.Zweier J L. J Biol Chem. 1988;263:1353–1357. [PubMed] [Google Scholar]
- 7.Kuzuya T, Hoshida S, Kim Y, Nishida M, Fuji H, Kitabatake A, Tada M, Kamada T. Circ Res. 1990;66:1160–1165. doi: 10.1161/01.res.66.4.1160. [DOI] [PubMed] [Google Scholar]
- 8.Ambrosio G, Zweier J L, Flaherty J T. J Mol Cell Cardiol. 1991;23:1359–1374. doi: 10.1016/0022-2828(91)90183-m. [DOI] [PubMed] [Google Scholar]
- 9.Henry T D, Archer S L, Nelson D, Weir E K, From A H L. Am J Physiol. 1993;264:H1478–H1484. doi: 10.1152/ajpheart.1993.264.5.H1478. [DOI] [PubMed] [Google Scholar]
- 10.Tosaki A, Bagchi D, Pali T, Cordis G A, Das D K. Biochem Pharmacol. 1993;45:961–969. doi: 10.1016/0006-2952(93)90182-v. [DOI] [PubMed] [Google Scholar]
- 11.Xia Y, Khatchikian G, Zweier J L. J Biol Chem. 1996;271:10096–10102. doi: 10.1074/jbc.271.17.10096. [DOI] [PubMed] [Google Scholar]
- 12.Esterbauer H, Schaur R J, Zollner H. Free Radical Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 13.Beckman K B, Ames B N. J Biol Chem. 1997;272:19633–19636. doi: 10.1074/jbc.272.32.19633. [DOI] [PubMed] [Google Scholar]
- 14.Berlett B S, Stadtman E R. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 15.Nohl H, Hegner D. Eur J Biochem. 1978;82:563–567. doi: 10.1111/j.1432-1033.1978.tb12051.x. [DOI] [PubMed] [Google Scholar]
- 16.Nohl H, Breuninger V, Hegner D. Eur J Biochem. 1978;90:385–390. doi: 10.1111/j.1432-1033.1978.tb12615.x. [DOI] [PubMed] [Google Scholar]
- 17.Hansford R G. Biochim Biophys Acta. 1983;726:41–80. doi: 10.1016/0304-4173(83)90010-1. [DOI] [PubMed] [Google Scholar]
- 18.Sawada M, Carlson J C. Mech Aging Dev. 1987;41:125–137. doi: 10.1016/0047-6374(87)90057-1. [DOI] [PubMed] [Google Scholar]
- 19.Sohal R J, Arnold L A, Sohal B H. Free Radical Biol Med. 1990;10:495–500. doi: 10.1016/0891-5849(90)90127-5. [DOI] [PubMed] [Google Scholar]
- 20.Das D K, George A, Liu X, Rao P S. Biochem Biophys Res Commun. 1989;165:1004–1009. doi: 10.1016/0006-291x(89)92702-2. [DOI] [PubMed] [Google Scholar]
- 21.Ambrosio G, Zweier J L, Duilio C, Kuppusamy P, Santoro G, Elia P P, Tritto I, Cirillo P, Condorelli M, Chiariello M, Flaherty J T. J Biol Chem. 1993;268:18532–18541. [PubMed] [Google Scholar]
- 22.Duan J, Karmazyn M. Can J Physiol Pharmacol. 1989;67:704–709. doi: 10.1139/y89-114. [DOI] [PubMed] [Google Scholar]
- 23.Hardy L, Clark J B, Darley-Usmar V M, Smith D R, Stone D. Biochem J. 1991;274:133–137. doi: 10.1042/bj2740133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karmazyn M. Can J Physiol Pharmacol. 1991;69:719–730. doi: 10.1139/y91-108. [DOI] [PubMed] [Google Scholar]
- 25.Veitch K, Hombroeckx A, Caucehteux D, Pouleur H, Hue L. Biochem J. 1991;281:709–715. doi: 10.1042/bj2810709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Jaarsveld H, Kuyl J M, Albert D W, Wiid M N. Res Commun Mol Pathol Pharmacol. 1994;85:33–44. [PubMed] [Google Scholar]
- 27.van Jaarsveld H, Kuyl J M, van Zyl G F, Barnard H C. Res Commun Mol Pathol Pharmacol. 1994;86:287–295. [PubMed] [Google Scholar]
- 28.Koller P T, Bergmann S R. Circ Res. 1989;65:838–846. doi: 10.1161/01.res.65.3.838. [DOI] [PubMed] [Google Scholar]
- 29.Ambrosio G, Flaherty J T, Duilio C, Tritto I, Santoro G, Elia P P, Condorelli M, Chiariello M. J Clin Invest. 1991;87:2056–2066. doi: 10.1172/JCI115236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tavazzi G, Lazzarino G, Di Pierro D, Giardina B. Free Radical Biol Med. 1992;13:75–78. doi: 10.1016/0891-5849(92)90167-f. [DOI] [PubMed] [Google Scholar]
- 31.Kramer J H, Misik V, Weglicki W B. Ann NY Acad Sci. 1994;723:180–196. [PubMed] [Google Scholar]
- 32.Blasig I E, Grune T, Schonheit K, Rohde E, Jakstadt M, Haseloff R F, Siems W G. Am J Physiol. 1995;268:H14–H22. doi: 10.1152/ajpheart.1995.269.1.H14. [DOI] [PubMed] [Google Scholar]
- 33.Cordis G A, Maulik N, Das D K. J Mol Cell Cardiol. 1995;27:1645–1653. doi: 10.1016/s0022-2828(95)90656-8. [DOI] [PubMed] [Google Scholar]
- 34.Pucheu S, Coudray C, Vanzetto G, Favier A, Machecourt J, de Leiris J. Free Radical Biol Med. 1995;19:873–881. doi: 10.1016/0891-5849(95)94361-g. [DOI] [PubMed] [Google Scholar]
- 35.Friguet B, Stadtman E R, Szweda L I. J Biol Chem. 1994;269:21639–21643. [PubMed] [Google Scholar]
- 36.Nadkarni D V, Sayre L M. Chem Res Toxicol. 1995;8:284–291. doi: 10.1021/tx00044a014. [DOI] [PubMed] [Google Scholar]
- 37.Tsai L, Sokoloski E A. Free Radical Biol Med. 1995;19:39–44. doi: 10.1016/0891-5849(95)00009-m. [DOI] [PubMed] [Google Scholar]
- 38.Cohn J A, Tsai L, Friguet B, Szweda L I. Arch Biochem Biophys. 1996;328:158–164. doi: 10.1006/abbi.1996.0156. [DOI] [PubMed] [Google Scholar]
- 39.Szweda L I, Uchida K, Tsai L, Stadtman E R. J Biol Chem. 1993;268:3342–3347. [PubMed] [Google Scholar]
- 40.Uchida K, Stadtman E R. J Biol Chem. 1993;268:6388–6393. [PubMed] [Google Scholar]
- 41.Chen J J, Bertrand H, Yu B P. Free Radical Biol Med. 1995;19:583–590. doi: 10.1016/0891-5849(95)00066-7. [DOI] [PubMed] [Google Scholar]
- 42.Humphries, K. M., Yoo, Y. & Szweda, L. I. (1998) Biochemistry, in press. [DOI] [PubMed]
- 43.Langendorff O. Arch Gesamte Physiol Mens Tiere. 1895;61:291–332. [Google Scholar]
- 44.Uchida K, Szweda L I, Chae H-Z, Stadtman E R. Proc Natl Acad Sci USA. 1993;90:8742–8746. doi: 10.1073/pnas.90.18.8742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Uchida K, Itakura K, Kawakishi S, Hiai H, Toyokuni S, Stadtman E R. Arch Biochem Biophys. 1995;324:241–248. doi: 10.1006/abbi.1995.0036. [DOI] [PubMed] [Google Scholar]
- 46.Reimer K A, Jennings R B. In: The Heart and Cardiovascular System. 2nd Ed. Fozzard H A, Haber E, Jennings R B, Katz A M, Morgan H E, editors. New York: Raven; 1992. pp. 1875–1973. [Google Scholar]