Abstract
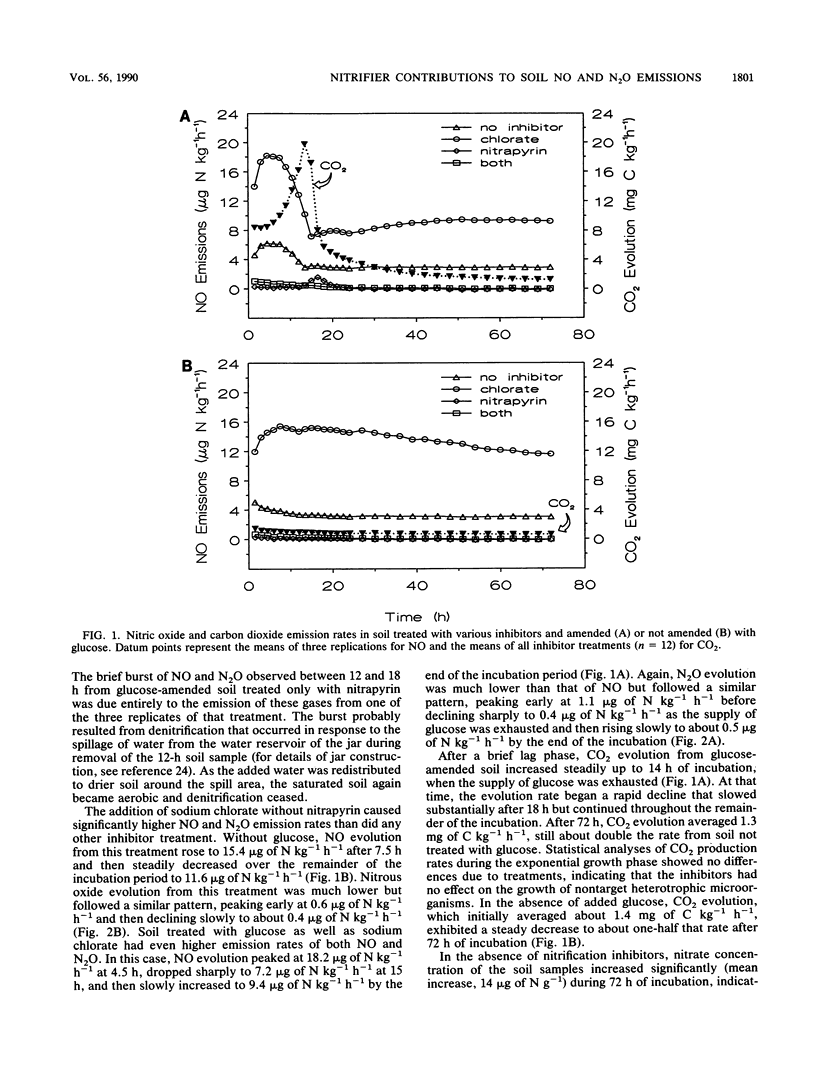
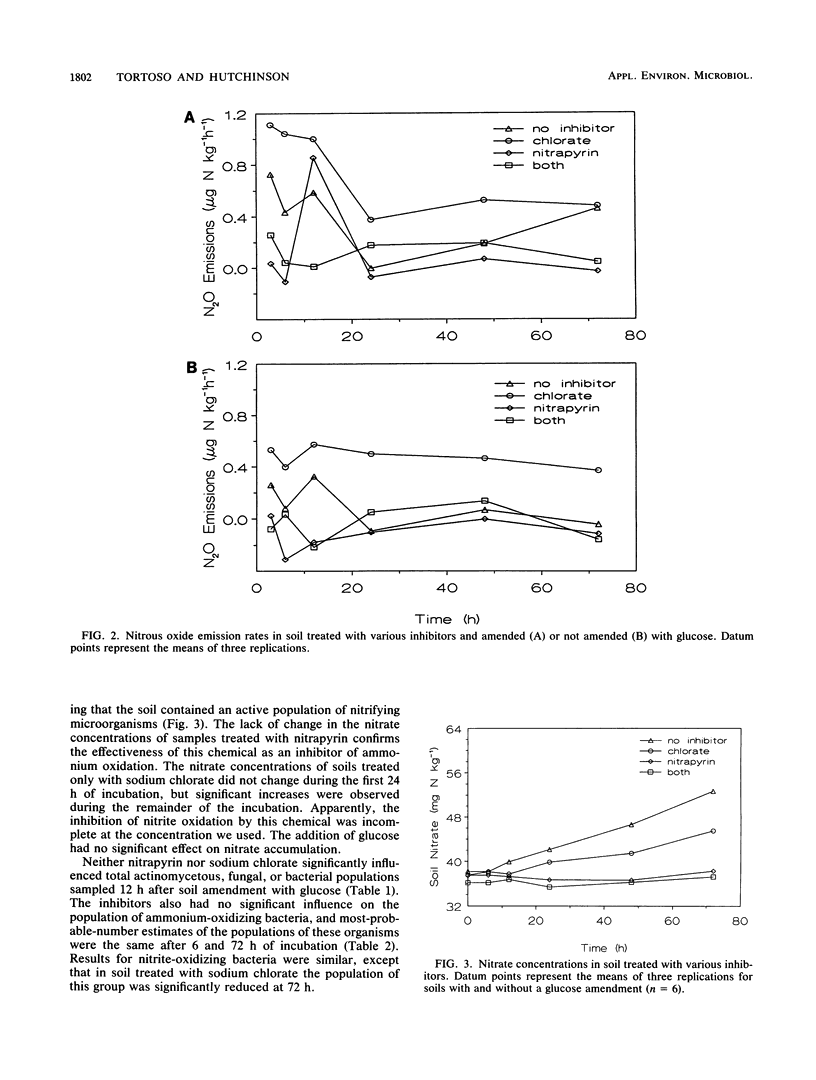
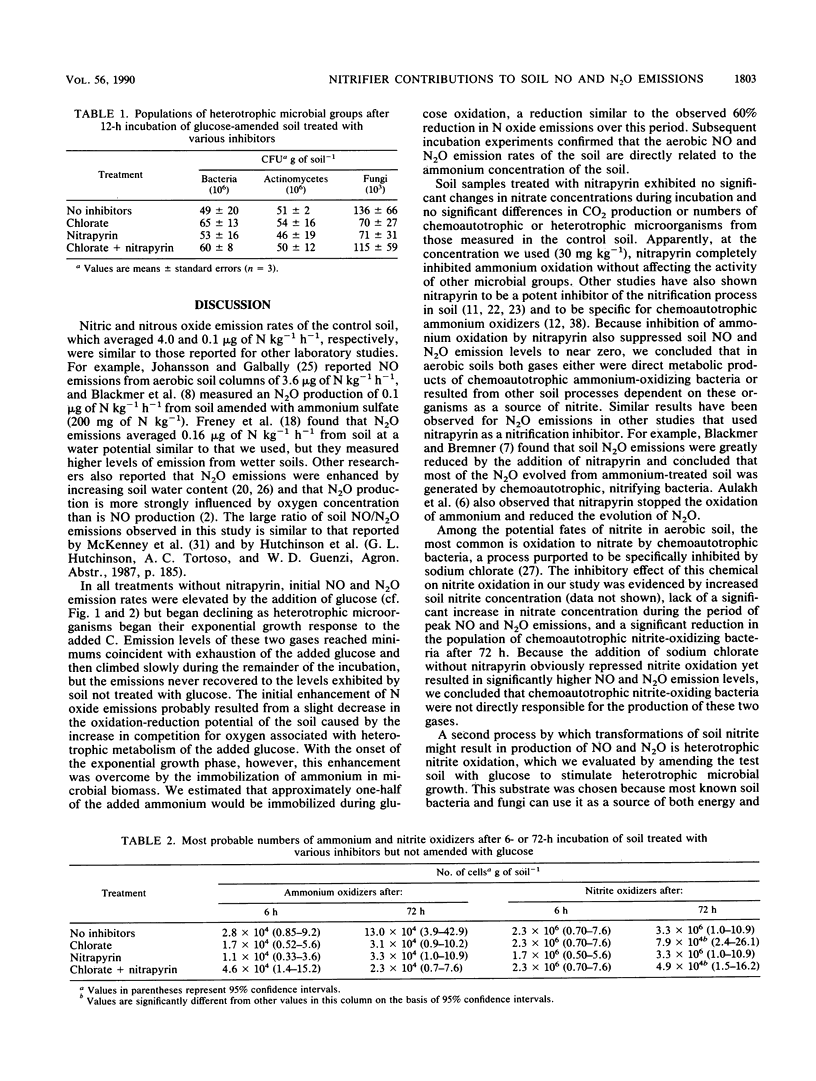
Soil emission of gaseous N oxides during nitrification of ammonium represents loss of an available plant nutrient and has an important impact on the chemistry of the atmosphere. We used selective inhibitors and a glucose amendment in a factorial design to determine the relative contributions of autotrophic ammonium oxidizers, autotrophic nitrite oxidizers, and heterotrophic nitrifiers to nitric oxide (NO) and nitrous oxide (N2O) emissions from aerobically incubated soil following the addition of 160 mg of N as ammonium sulfate kg−1. Without added C, peak NO emissions of 4 μg of N kg−1 h−1 were increased to 15 μg of N kg−1 h−1 by the addition of sodium chlorate, a nitrite oxidation inhibitor, but were reduced to 0.01 μg of N kg−1 h−1 in the presence of nitrapyrin [2-chloro-6-(trichloromethyl)-pyridine], an inhibitor of autotrophic ammonium oxidation. Carbon-amended soils had somewhat higher NO emission rates from these three treatments (6, 18, and 0.1 μg of N kg−1 h−1 after treatment with glucose, sodium chlorate, or nitrapyrin, respectively) until the glucose was exhausted but lower rates during the remainder of the incubation. Nitrous oxide emission levels exhibited trends similar to those observed for NO but were about 20 times lower. Periodic soil chemical analyses showed no increase in the nitrate concentration of soil treated with sodium chlorate until after the period of peak NO and N2O emissions; the nitrate concentration of soil treated with nitrapyrin remained unchanged throughout the incubation. These results suggest that chemoautotrophic ammonium-oxidizing bacteria are the predominant source of NO and N2O produced during nitrification in soil.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson I. C., Levine J. S. Relative rates of nitric oxide and nitrous oxide production by nitrifiers, denitrifiers, and nitrate respirers. Appl Environ Microbiol. 1986 May;51(5):938–945. doi: 10.1128/aem.51.5.938-945.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. P., Domsch K. H. Measurement of bacterial and fungal contributions to respiration of selected agricultural and forest soils. Can J Microbiol. 1975 Mar;21(3):314–322. doi: 10.1139/m75-045. [DOI] [PubMed] [Google Scholar]
- Blackmer A. M., Bremner J. M., Schmidt E. L. Production of nitrous oxide by ammonia-oxidizing chemoautotrophic microorganisms in soil. Appl Environ Microbiol. 1980 Dec;40(6):1060–1066. doi: 10.1128/aem.40.6.1060-1066.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAMPBELL N. E., ALEEM M. I. THE EFFECT OF 2-CHLORO, 6-(TRICHLOROMETHYL) PYRIDINE ON THE CHEMOAUTOTROPHIC METABOLISM OF NITRIFYING BACTERIA. II. NITRITE OXIDATION BY NITROBACTER. Antonie Van Leeuwenhoek. 1965;31:137–144. doi: 10.1007/BF02045883. [DOI] [PubMed] [Google Scholar]
- Castignetti D., Gunner H. B. Sequential nitrification by an Alcaligenes sp. and Nitrobacter agilis. Can J Microbiol. 1980 Sep;26(9):1114–1119. doi: 10.1139/m80-184. [DOI] [PubMed] [Google Scholar]
- Davidson E. A., Swank W. T., Perry T. O. Distinguishing between Nitrification and Denitrification as Sources of Gaseous Nitrogen Production in Soil. Appl Environ Microbiol. 1986 Dec;52(6):1280–1286. doi: 10.1128/aem.52.6.1280-1286.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goreau T. J., Kaplan W. A., Wofsy S. C., McElroy M. B., Valois F. W., Watson S. W. Production of NO(2) and N(2)O by Nitrifying Bacteria at Reduced Concentrations of Oxygen. Appl Environ Microbiol. 1980 Sep;40(3):526–532. doi: 10.1128/aem.40.3.526-532.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward E., Whitwell H., Paul K. S., Barnes D. M. Steroid receptors in human meningioma. Clin Neuropharmacol. 1984;7(4):351–356. doi: 10.1097/00002826-198412000-00014. [DOI] [PubMed] [Google Scholar]
- Johansson C., Galbally I. E. Production of nitric oxide in loam under aerobic and anaerobic conditions. Appl Environ Microbiol. 1984 Jun;47(6):1284–1289. doi: 10.1128/aem.47.6.1284-1289.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEES H., SIMPSON J. R. The biochemistry of the nitrifying organisms. V. Nitrite oxidation by Nitrobacter. Biochem J. 1957 Feb;65(2):297–305. doi: 10.1042/bj0650297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Jones D., Tokarewicz A., Watson P. G. Clinical evaluation of clobetasone butyrate eye drops in episcleritis. Br J Ophthalmol. 1981 Sep;65(9):641–643. doi: 10.1136/bjo.65.9.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenney D. J., Shuttleworth K. F., Vriesacker J. R., Findlay W. I. Production and loss of nitric oxide from denitrification in anaerobic brookston clay. Appl Environ Microbiol. 1982 Mar;43(3):534–541. doi: 10.1128/aem.43.3.534-541.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroo H. F., Klein T. M., Alexander M. Heterotrophic nitrification in an Acid forest soil and by an Acid-tolerant fungus. Appl Environ Microbiol. 1986 Nov;52(5):1107–1111. doi: 10.1128/aem.52.5.1107-1111.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace W., Nicholas D. J. The biochemistry of nitrifying microorganisms. Biol Rev Camb Philos Soc. 1969 Jul;44(3):359–391. doi: 10.1111/j.1469-185x.1969.tb01216.x. [DOI] [PubMed] [Google Scholar]



