Abstract
Background
Macrosomia is associated with considerable neonatal and maternal morbidity. Factors that predict macrosomia are poorly understood. The increased rate of macrosomia in the offspring of pregnant women with diabetes and in congenital hyperinsulinaemia is mediated by increased foetal insulin secretion. We assessed the in utero and neonatal role of two key regulators of pancreatic insulin secretion by studying birthweight and the incidence of neonatal hypoglycaemia in patients with heterozygous mutations in the maturity-onset diabetes of the young (MODY) genes HNF4A (encoding HNF-4α) and HNF1A/TCF1 (encoding HNF-1α), and the effect of pancreatic deletion of Hnf4a on foetal and neonatal insulin secretion in mice.
Methods and Findings
We examined birthweight and hypoglycaemia in 108 patients from families with diabetes due to HNF4A mutations, and 134 patients from families with HNF1A mutations. Birthweight was increased by a median of 790 g in HNF4A-mutation carriers compared to non-mutation family members (p < 0.001); 56% (30/54) of HNF4A-mutation carriers were macrosomic compared with 13% (7/54) of non-mutation family members (p < 0.001). Transient hypoglycaemia was reported in 8/54 infants with heterozygous HNF4A mutations, but was reported in none of 54 non-mutation carriers (p = 0.003). There was documented hyperinsulinaemia in three cases. Birthweight and prevalence of neonatal hypoglycaemia were not increased in HNF1A-mutation carriers. Mice with pancreatic β-cell deletion of Hnf4a had hyperinsulinaemia in utero and hyperinsulinaemic hypoglycaemia at birth.
Conclusions
HNF4A mutations are associated with a considerable increase in birthweight and macrosomia, and are a novel cause of neonatal hypoglycaemia. This study establishes a key role for HNF4A in determining foetal birthweight, and uncovers an unanticipated feature of the natural history of HNF4A-deficient diabetes, with hyperinsulinaemia at birth evolving to decreased insulin secretion and diabetes later in life.
HNF4A mutations were found to be associated with a considerable increase in birthweight and macrosomia, and were a cause of neonatal hypoglycaemia.
Editors' Summary
Background.
MODY, or maturity-onset diabetes of the young, is a particular subtype of diabetes; only a few percent of people with diabetes are thought to have this subtype. The condition comes about as a result of a mutation in one of six genes. Generally, people with MODY have high glucose (sugar) levels in the blood, and the typical symptoms of diabetes, such as increased thirst and urination, typically develop when the person is below the age of 25 y. Two of the genes that are known to cause MODY are mutant forms of HNF4A and HNF1A. The proteins that are encoded by these two genes control insulin levels produced by the pancreas; when these genes are mutated, not enough insulin is produced. Without enough insulin to control blood sugar, levels rise, leading to the symptoms of diabetes. However, MODY can be managed by many of the same interventions as other types of diabetes, such as diet, exercise, drug treatments, and insulin injections.
Why Was This Study Done?
Although the evidence shows that individuals who carry mutations in HNF4A and HNF1A do not produce enough insulin and therefore have higher glucose levels in their blood, there were some tantalizing suggestions from mouse experiments that this might not be the whole story. Specifically, the researchers suspected that during embryonic development, mutations in HNF4A or HNF1A might actually cause higher insulin levels. Too much insulin during development of a fetus is known to cause it to gain weight, resulting in a baby that is larger than the average size for its age. Larger babies are risky for both the baby and the mother. The researchers doing this study wanted to understand more precisely what the links were between the forms of MODY caused by HNF4A and HNF1A mutations, and birth-weight and blood-sugar levels.
What Did the Researchers Do and Find?
In this study, the researchers examined 15 families in which some family members had MODY caused by a mutation in HNF4A. They compared the birthweight for family members carrying the mutation (54 people) against the birthweight for those who did not (54 people). A similar comparison was done for 38 families in which some members had a different form of MODY, this time caused by a mutation in HNF1A. The results showed that the birthweight of family members who carried a mutation in HNF4A was, on average, 790 g higher than the birthweight of family members who didn't carry the mutation. Low blood-sugar levels at birth were also more common in people carrying the HNF4A mutation as compared to people who did not. However, the HNF1A mutation did not seem to be associated with greater birthweight or low blood-sugar levels at birth. Finally, in order to understand these findings further, the researchers created embryonic mice carrying mutations in the mouse equivalent of HNF4A. These embryos produced more insulin than normal mouse embryos and, after birth, were more likely to have low blood-sugar levels.
What Do These Findings Mean?
These findings show that there is a link between mutations in HNF4A, but not in HNF1A, and increased birthweight. The increase found in this study is quite substantial (a median weight of 4,660 g in the affected babies; a birthweight of more than 4,000 g is generally considered large). The results suggest that in human embryos with a mutated form of HNF4A, too much insulin is produced during development, causing faster growth and a higher chance of the baby being born with low blood-sugar levels. This is an unexpected finding, because later in life the HNF4A mutation causes lower insulin levels. Therefore, the biochemical pathways causing this type of MODY seem to be quite complicated, and further research will need to be done to fully understand them. Crucially, the research also suggests that pregnant women carrying HNF4A mutations should be closely followed to check their baby's growth and minimize the chance of complications. Doctors and families should also consider doing a genetic test for HNF4A if a baby has low blood-sugar levels and if there is a family history of diabetes; this would increase the chance of diagnosing MODY early.
Additional Information.
Please access these Web sites via the online version of this summary at http://dx.doi.org/10.1371/journal.pmed 0040118.
In a related Perspective in PLoS Medicine, Benjamin Glaser discusses causes of type 2 diabetes mellitus in the context of this study's findings
The US National Institute of Diabetes and Digestive and Kidney Diseases has pages of information on different types of diabetes
Wikipedia has an entry on Maturity Onset Diabetes of the Young (MODY) (note that Wikipedia is an internet encyclopedia that anyone can edit)
Diabetes Research Department, Peninsula Medical School, Exeter, UK provides information for patients and doctors on genetic types of diabetes; the website is maintained by the research group carrying out this study
Information from the Centers for Disease Control and Prevention on diabetes and pregnancy
Introduction
Macrosomia is associated with considerable foetal and maternal morbidity [1]. Factors that predict macrosomia are still poorly understood [2]. In humans, foetal insulin secretion is one of the key determinants of foetal growth, acting mainly in the third trimester when the weight of the foetus increases greatly. This is seen in pregnant women with diabetes when foetal sensing of maternal hyperglycemia drives insulin secretion, insulin-mediated growth, and subsequent macrosomia. In addition to such environmental factors, mutations in the genes involved in insulin secretion are also known to affect birthweight. Mutations that cause hyperinsulinaemic hypoglycaemia of infancy [3–10] are associated with increased birthweight. Conversely, genes in which mutations cause neonatal diabetes [11,12] and some forms of maturity-onset diabetes of the young (MODY) [13,14] are associated with decreased birthweight.
The transcription factors hepatocyte nuclear factor-4α (encoded by the HNF4A gene), and hepatocyte nuclear factor-1α (encoded by HNF1A, approved gene name TCF1) play a key role in the regulation of pancreatic insulin secretion. HNF4A and HNF1A mutations cause monogenic diabetes (MODY 1 and MODY3, respectively) due to decreased insulin secretion [15,16] and are key parts of an important β-cell network [17,18]. In the pancreas, HNF1A and HNF4A form part of a common transcriptional network, which has been proposed as an explanation of the shared pancreatic phenotype seen in patients with mutations in these genes [18]. Variants in the HNF4A pancreatic promoter have also been associated with Type 2 diabetes [19,20].
A recent study reported mildly reduced blood glucose and increased insulin levels in adult β-cell Hnf4a–deficient mice [21]. As a result of this animal study, we hypothesized that mutations in the human gene HNF4A might increase foetal insulin secretion and birthweight, and cause neonatal hyperinsulinaemia and hypoglycaemia. We therefore studied birthweight and reported hypoglycaemia in HNF4A-mutation carriers and unaffected family members. As a comparison, we also studied the families of patients with mutations in the closely associated pancreatic transcription factor HNF1A. To investigate the mechanism of neonatal hypoglycaemia and increased birthweight, we also studied foetal and neonatal mice lacking both copies of Hnf4a in the pancreas.
Methods
Birthweight and Reported Hypoglycaemia in Hnf4a-Mutation Carriers
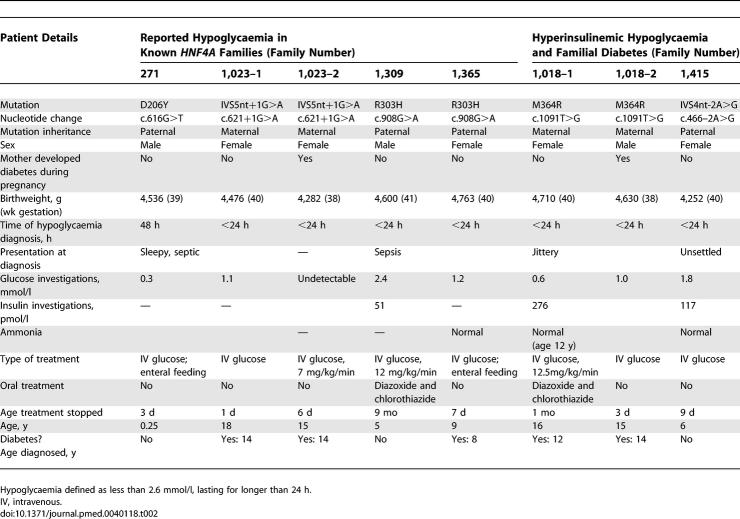
One hundred and eight members (54 mutation carriers) of 15 families who had been found to have MODY due to an HNF4A mutation, were contacted. This group included 13 families where the mutation had previously been identified as well as two families, found after screening for HNF4A, where there was both neonatal hypoglycaemia and diabetes in a family member (see below). Where mutation status of an individual within a family had not previously been determined, DNA was extracted from a buccal sample. HNF4A was amplified and sequenced as previously described [22]. Birthweight and gestational age were primarily obtained by maternal recall. Birth centiles and weight were corrected to 40 wk of gestation and for male sex, according to UK 1990 reference curves [23].
In assessment of neonatal hypoglycaemia, we report here on three patients from two families described above in whom HNF4A mutations had been identified due to coexistent familial diabetes and neonatal hypoglycaemia. In addition, we contacted 101 unselected members (48 mutation carriers) of the 13 families described above who had been found previously to have MODY due to an HNF4A mutation. In babies born to mothers with diabetes during pregnancy, hypoglycaemia that did not require intravenous glucose and lasted for less than 24 h was not considered exceptional. Any other reported incidence of hypoglycaemia at birth was followed up by case-note review. An episode of hypoglycaemia was established only if venous plasma glucose of less than 2.5 mmol/l was documented. All investigations for hyperinsulinaemic hypoglycaemia were done by the referring clinician at the time of diagnosis using their local laboratory.
HNF4A Mutations in Families with both Diabetes and Hypoglycaemia
After our initial observations in families with known HNF4A mutations, we went on to sequence the HNF4A gene in the probands of five further families who had been referred to the Exeter Molecular Genetics Laboratory with hyperinsulinaemic hypoglycaemia, and who had at least one first-degree relative with diabetes. No monogenic cause had been found for the hypoglycaemia or diabetes in these families: three of the hypoglycaemic probands had been sequenced for activating GCK and/or KCNJ11 mutations and, in one family, members with diabetes had been sequenced for HNF1A mutations.
Birthweight and Reported Hypoglycaemia in HNF1A-Mutation Carriers
One hundred and thirty-four members (85 mutation carriers) from 38 families with known MODY due to an HNF1A mutation were contacted. Reported birthweight and hypoglycaemia were recorded in the same way as for HNF4A-mutation carriers.
Transgenic Mice
β-cell–specific Hnf4a mutations (β-Hnf4a-KO) were generated by crossing Hnf4a LoxP/LoxP mice (Jackson Laboratory, http://www.jax.org) [24] with InsPr-Cre mice, a transgenic line expressing Cre recombinase driven by the rat insulin 2 promoter [25]. Both lines were bred on a C57BL/6J genetic background. An almost complete efficiency of recombination of Hnf4a LoxP alleles in β-cells was verified by: (1) real-time PCR quantitation in RNA from isolated islets showing an 80% reduction of Hnf4a mRNA relative to control mice (Figure S1); (2) absent Hnf-4α staining in >90% β-cells on immunofluroescence analysis (unpublished data); and (3) recombination of a Rosa26LoxP-Stop-LoxP-Lacz allele in >90% of β-cells when crossed with InsPr-Cre (unpublished data). Blood insulin and glucose were obtained after decapitation of timed embryos or neonatal mice (15–20 mice per genotype and stage). Glucose was measured with a glucose meter (Accu-Chek, Roche [http://www.roche.com]). Plasma insulin levels were determined by ELISA (Mercodia, http://www.mercodia.com). Genotyping was performed by PCR analysis using genomic DNA isolated from the tail tips of embryos and newborn mice.
RNA Analysis
Isolated islets from 6–8-wk-old β-HNF4a-KO mice and control Hnf4a LoxP/LoxP littermates were used for RNA extraction with Trizol reagent (Invitrogen, http://www.invitrogen.com). RNA integrity was verified with the 2100 Bioanalyzer (Agilent Technologies, http://www.agilent.com) prior to reverse transcription and real-time PCR quantitation as described [26]. Oligonucleotide sequences are available upon request.
Statistical Analysis
Owing to the non-normal distribution of birthweight data, non-parametric analysis was used. Median centiles and birthweights were compared using the Mann-Whitney U-test. For the discordant sibling analysis, the median birthweight corrected for sex and gestation for all mutation carriers and non-mutation carriers within a sibship were compared using the Wilcoxon signed rank test. Hypoglycaemia and macrosomia categorical data were compared using Fisher's Exact test. Data from the mouse studies are presented as mean ± standard error of the mean, and were compared by the two-tailed Student's t-test.
Ethical Approval
This study was approved by the North and East Devon Regional Ethics Committee, UK and the Animal Ethics Committee of the University of Barcelona School of Medicine, Spain. All patients or carers gave informed consent.
Results
Birthweight in HNF4A-Mutation Carriers
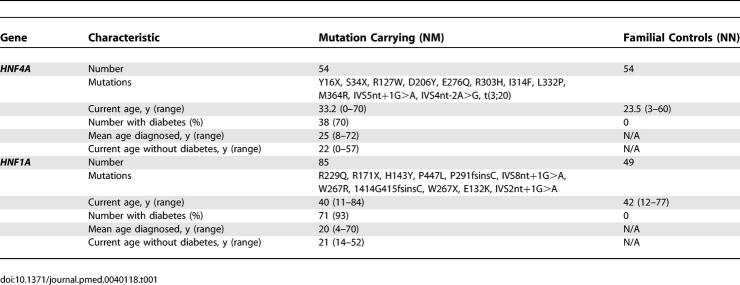
The characteristics of all the HNF4A-mutation carriers and their unaffected family members are shown in Table 1. The median birthweight of the HNF4A-mutation carriers was the 96th centile (interquartile range 75–100) compared with the 58th centile (interquartile range 33–76) in unaffected family members, giving a difference in corrected median birthweight of 790 g, p < 0.001 (Figure 1A). A difference in birthweight was seen both when the mutation was inherited from the father, p < 0.001 (Figure 1B) or from the mother, p < 0.001 (Figure 1C). There was no effect of offspring genotype on gestational age at delivery (p = 0.29). The influence of HNF4A genotype on birthweight remained significant if the individuals from the two families referred with hyperinsulinaemia were excluded (p < 0.001). Nineteen mothers who were mutation carriers and one mother who was not a mutation carrier had diabetes during pregnancy; 11 of the 20 offspring from these pregnancies were mutation carriers. Finally, to allow for any impact of maternal hyperglycaemia, corrected median birthweight was compared in 18 sibling pairs discordant for the presence of the HNF4A mutation. The median birthweight of the mutation-carrying siblings was 4,660 g compared with a median birthweight of the non-mutation-carrying siblings of 3,640 g, p = 0.001 (Figure 1D).
Table 1.
Characteristics of Patients
Figure 1. Birthweight (Adjusted for Sex and Gestational Age) according to Foetal Genotype.
(A) All offspring; (B) HNF4A mutation inherited from the father; (C) HNF4A mutation inherited from the mother, and (D) siblings discordant for HNF4A genotype.
Foetal genotype: NM, heterozygous HNF4A mutation; NN, normal HNF4A. For (A) to (C), bars represent median, the box represents interquartile range, and the whiskers represent the range, with outliers shown as circles. Comparing NM birthweight with NN birthweight by Mann-Whitney U-test: p < 0.001 for (A) all offspring; p = 0.001 for (B) father affected and for (C) mother affected. In (D), those pairs where the father has the mutation are shown as filled circles; those pairs where the mother has the mutation are shown as filled triangles. The red bars represent median birthweight.
Macrosomia, defined as a birthweight of more than 4,000 g, was present in 56% of HNF4A-mutation carriers but in only 13% of non-mutation carriers (p < 0.001). The prevalence of macrosomia was 64% if the HNF4A mutation was inherited from the mother and 46% if the HNF4A mutation was inherited from the father. In contrast in an unaffected foetus, the equivalent rates were 25% with an affected mother (p = 0.07), and 6% with an affected father (p = 0.003). Macrosomia is associated with increased foetal and maternal morbidity, and this was seen in some of the patients. The deliveries of the two siblings from family 1,023 were both complicated by severe shoulder dystocia, with 1,023–1 developing an Erb's palsy. The prevalence of extreme macrosomia, defined as a birthweight of >5,000 g, which is associated with increased neonatal mortality [27], was 15% (four neonates) in HNF4A-mutation carriers with an affected mother and 7% (two neonates) in HNF4A-mutation carriers with an affected father. No non-mutation carriers had extreme macrosomia.
Neonatal Hyperinsulinaemic Hypoglycaemia in HNF4A-Mutation Carriers
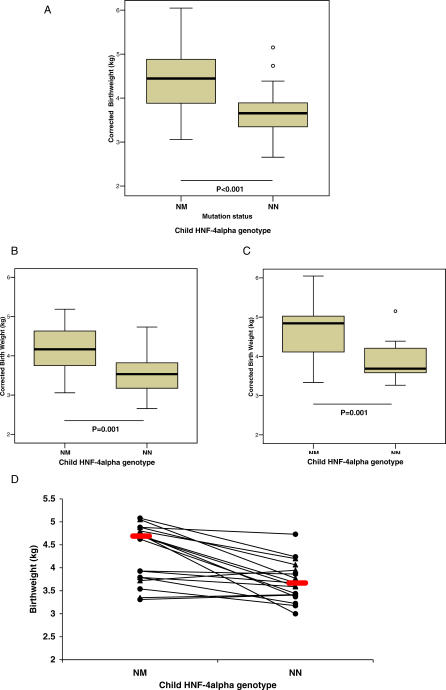
Transient neonatal hypoglycaemia is a feature of some HNF4A-mutation carriers. Two out of five families referred to Exeter, with hypoglycaemia and a first-degree family member with diabetes, were shown to have novel HNF4A mutations (M364R, IVS4nt-2A>G). Three out of the six mutation carriers in these families had documented neonatal hypoglycaemia (Figure 2; Table 2). In addition, five out of 48 HNF4A-mutation carriers in families previously identified with MODY had hypoglycaemia at or soon after birth lasting > 24 h and requiring treatment (Table 2). So, overall, eight out of 54 mutation carriers had neonatal hypoglycaemia compared to none of the 54 non-mutation carriers (p = 0.003). If the patients from the two additional families are excluded, there is still a significant excess of hypoglycaemia in the mutation carriers (p = 0.02).
Figure 2. Pedigrees of Families with Hypoglycaemia.
Patients with hypoglycaemia are shaded with bold diagonal stripes; patients who had hypoglycaemia but have progressed onto diabetes are shaded with half black/half diagonal stripes; and patients with diabetes are coloured black. Probands are indicated with an arrow. Where available, below each symbol is recorded genotype (NM, HNF4A-mutation carrier; NN, unaffected), birth centile adjusted for sex and gestation, age at which diabetes developed, and the patient's treatment.
Table 2.
Clinical Features of Patients with HNF4A Mutations and Documented Hypoglycaemia
The clinical features of the eight cases with established hypoglycaemia during infancy associated with HNF4A mutations are shown in Table 2. Their pedigrees (Figure 2) show that hypoglycaemia was often described only in a single family member with other carriers of the same HNF4A mutation presenting with diabetes. The prevalence of hypoglycaemia was similar if the mutation was inherited from the father (four out of 27 patients) or from the mother (four out of 27 patients), suggesting that persisting neonatal hypoglycaemia is independent of maternal glycaemia in pregnancy. In six patients, the hypoglycaemia was treated with intravenous glucose and enteral feeding, but two patients (1,018–1 and 1,309) required treatment with diazoxide and chlorothiazide for 1 and 6 mo, respectively. In both cases, there was documented inappropriate hyperinsulinaemia in the presence of hypoglycaemia (Table 2).
β-Cell Deletion of Hnf4a in Mice
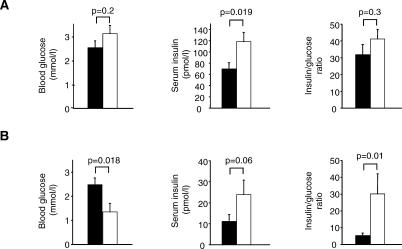
To directly test whether Hnf4a deficiency affects insulin secretion in utero, mice with β-cell–specific Hnf4a deletion (β-Hnf4a-KO) were examined during late gestation (E18.5–E20). Late-gestation β-Hnf4a-KO embryos exhibited significantly elevated insulin concentrations (118.14 ± 16.62 pmol/l versus 69.75 ± 11.03 pmol/l in β-Hnf4a-KO versus controls, p = 0.019) (Figure 3A). Glucose concentrations, which are regulated by the mother, were similar in both groups (Figure 3A). Birthweight, which in mice does not exhibit insulin dependence as in humans [28], did not differ in β-Hnf4a-KO mice. During the neonatal period, blood glucose values were low relative to control littermates (1.35 ± 0.35 mmol/l versus 2.50 ± 0.28 mmol/l, respectively, p = 0.018) (Figure 3B). Blood glucose levels below 1.1 mmol/l were observed at least seven times more frequently in neonatal β-Hnf4a-KO neonatal mice than in controls (8/15 versus 2/28, respectively, p < 0.001). This hypoglycaemia was due to increased insulin secretion, as the insulin/glucose ratio values in β-Hnf4a-KO versus control mice were 29.8 ± 12.0 versus 5.6 ± 1.5 (p = 0.01), while insulin values were 23.9 ± 6.9 pmol/l versus 11.1 ± 3.3 pmol/l (p = 0.053), respectively (Figure 3B). The expression of genes causing human hyperinsulinaemic hypoglycaemia, namely Kcnj11 (encoding Kir6.2), Abcc8 (encoding Sur1), Schad, Gck, and Glud1, was unaltered in Hnf4a-deficient islets (Figure S1). In summary, these findings indicate that Hnf4a deficiency causes hyperinsulinism during foetal and neonatal life, supporting the suggestion that this is the underlying cause of macrosomia and hypoglycaemia in HNF4A-mutation carriers.
Figure 3. Deletion of Hnf4a in β-Cells Results in Hyperinsulinaemia and Hypoglycaemia in Mice.
(A) Blood glucose, plasma insulin, and insulin/glucose ratio of E18.5–E20 embryos. (B) Blood glucose levels, plasma insulin levels, and insulin/glucose ratio of newborn mice. Data from β-Hnf4a-KO and controls is shown in white and black bars, respectively. Values are mean ± standard error of the mean.
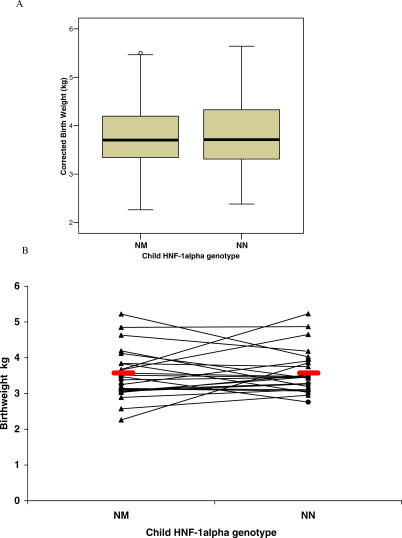
Birthweight and Hypoglycaemia in HNF1A-Mutation Carriers
The characteristics of the HNF1A-mutation carriers and their unaffected family members are shown in Table 1. Unlike HNF4A, mutations in HNF1A are not associated with an increased birthweight (Figure 4A), with a median difference of 10 g (p = 0.86) and a mean difference in the analysis of 24 discordant sibling-pairs (Figure 4B) of 3g (p = 0.91). Only one HNF1A-mutation carrier had neonatal hypoglycaemia requiring intravenous glucose and persisting for longer than 24 h; however, his mother had diabetes during pregnancy, and he required less than 48 h of this treatment. Hypoglycaemia was more common in HNF4A-mutation carriers (eight out of 54) than in HNF1A-mutation carriers (one out of 77), p = 0.004.
Figure 4. Birthweight in HNF1A-Mutation Carriers.
(A) Median centile (adjusted for sex and gestation) in mutation carriers (NM) and non-mutation carriers (NN) (p = 0.86). Error bars show interquartile range. (B) Discordant sib-pair analysis. Those pairs where the father has the mutation are shown as filled circles; those pairs where the mother has the mutation are shown as filled triangles. Median birthweight: NM 3,490 g; NN 3460 g (p = 0.91).
Discussion
We have shown that increased birthweight and macrosomia are common features of patients with HNF4A mutations and, in addition, that some individuals with HNF4A have neonatal hypoglycaemia. Although in young adults the same genetic defect results in diabetes due to reduced insulin secretion [15,22], we have shown that the mechanism for the phenotype in newborns is likely to be increased insulin secretion in utero and in the neonatal period. This is supported by hyperinsulinaemia in some affected infants with HNF4A mutations, and studies in mice with β-cell deletion of Hnf4a clearly show hyperinsulinaemia in utero and hyperinsulinaemic hypoglycaemia in the early neonatal period.
HNF4A-mutation carriers are, on average, 790 g heavier than their family members who do not carry the mutation, and 56% are born with macrosomia (>4,000 g). The increase in birthweight is similar to that seen in the offspring of patients with maternal diabetes which is the commonest recognized cause of macrosomia. Forty-six percent of children with an HNF4A mutation born to affected fathers had macrosomia. This is a clear example that macrosomia may result from foetal genetics as well as from the maternal intra-uterine environment. Consideration of this should be taken into account when determining macrosomia risk, and we recommend that, in addition to maternal diabetes, a history of young-onset non-insulin-requiring paternal diabetes should prompt assessment of foetal size.
We have described eight patients with HNF4A mutations who had one or more episodes of hypoglycaemia in the neonatal period; there was hyperinsulinaemia in all three patients who were tested. Five patients required treatment with intravenous glucose only, with resolution within 1 mo; this finding was consistent with a transient hyperinsulinaemia. Two patients had more persistent hypoglycaemia which responded well to treatment with diazoxide and chlorothiazide and subsequently resolved. Therefore, the loss of HNF4A function causes a relatively mild form of hyperinsulinaemic hypoglycaemia that is transient and diazoxide-responsive. Transient hypoglycaemia is often not investigated and as a result is understudied. We propose that neonates presenting with hypoglycaemia who have a father with diabetes, or a mother with young-onset non-insulin-requiring diabetes, should be screened for HNF4A mutations. However, three out of the five unselected HNF4A-mutation carriers with neonatal hypoglycaemia presented before their respective parent developed diabetes. Therefore, we also suggest that HNF4A mutations should be considered in any child with persistent hypoglycaemia (>24 h).
We encountered two problems resulting from the retrospective nature of this study. Firstly, hospital records were not readily available so birthweight and gestational age were ascertained by parental recall in the majority of cases. However, all this data collection was done blind to genotype and therefore any error should apply to both offspring groups. Secondly, the hypoglycaemia was often not well investigated at the time of presentation, presumably because of its transient nature. Hence, hyperinsulinaemia at birth was looked for only in three out of the eight patients presenting with hypoglycaemia, and other causes of hyperinsulinaemia were not excluded. It also means that we are uncertain whether the 46 patients with HNF4A mutations in whom hypoglycaemia was not described had undetected hypoglycaemia or were not hypoglycaemic. A prospective study of neonates born to HNF4A-mutation carriers is required for a complete assessment of the hyperinsulinaemic hypoglycaemia seen in these patients.
The increased birthweight and risk of macrosomia in HNF4A-mutation carriers is likely to be secondary to foetal hyperinsulinaemia. Although no measures of foetal insulin or cord insulin were available to confirm this mechanism in humans, two lines of evidence support it. Firstly, in humans, we have documented hyperinsulinaemia soon after birth in the three patients in whom it was tested, and hypoglycaemia in eight. Secondly, we have shown that mice lacking pancreatic Hnf4a have increased insulin concentrations in utero, and hyperinsulinaemic hypoglycaemia as newborns.
The increased birthweight and neonatal hypoglycaemia in HNF4A-mutation carriers seems paradoxical for a gene that is associated with a β-cell–deficient form of young-onset diabetes [15,22], particularly as the decreased β-cell function has been explained by decreased expression of pancreatic β-cell genes involved in glucose metabolism [29,30]. It is in contrast to other monogenic causes of diabetes—for example, GCK [13], IPF1 [31], HNF1B [14], or activating KCNJ11 [32,33] and ABCC8 mutations [12,34] where birthweight is reduced. In these cases, the low insulin secretion that causes diabetes later is associated with decreased insulin-mediated foetal growth. In HNF4A-mutation carriers, in contrast, there would need to be a switch from increased insulin secretion in utero and neonatal life to decreased insulin secretion in later life. The closest example of this is patients with hyperinsulinaemia of infancy due to recessive and dominant mutations in KATP channel subunits, who have a high rate of diabetes at long-term follow-up even when they do not receive pancreatic surgery [8,35,36]. It has been postulated that diabetes in SUR1-deficient patients reflects increased apoptosis, in addition to abnormal regulation of secretion due to lack of KATP channels [37]. Compared to SUR1 deficiency, HNF-4α deficiency results in less severe hyperinsulinism, yet gives rise to a more highly penetrant and severe diabetic phenotype. It is interesting, however, that of the eight patients who developed established hypoglycaemia at birth, five developed diabetes by the age of 14 y (mean age 12.4 y), suggesting a possible earlier progression to diabetes in this group.
Two recent studies surprisingly showed that β-cell Hnf4a deficiency does not cause diabetes in mice [21,38]. One study paradoxically reported mildly reduced blood glucose and increased insulin levels in adult β-cell Hnf4a-deficient mice, and ascribed this to diminished expression of Kcnj11 encoding the KATP channel subunit Kir6.2 [21]. Another study failed to confirm abnormal blood glucose and insulin levels, and reported normal Kcnj11 expression [38]. This discrepancy, together with the unexpected failure to develop hypoinsulinaemic diabetes, led us to question whether hyperinsulinaemia was an important feature of Hnf4a deficiency. In the current study, we have shown that, in parallel with the human findings, Hnf4a–deficient mice exhibit hyperinsulinaemia in the foetal and neonatal stage, as well as overt neonatal hypoglycaemia as opposed to only mildly reduced glucose at later ages as recently reported [21]. Importantly, our studies showed no abnormal expression of Kcnj11. While discrepancies in phenotype might be explained by small differences in genetic background, the current data suggest that the hyperinsulinaemic phenotype in Hnf4a deficiency is not related to KATP channel expression.
Further studies will need to address how Hnf-4α-dependent transcription in β-cells is linked to the dual phenotype reported here. Large-scale profiling shows that Hnf-4α-deficient β-cells exhibit abnormal expression of more than 10% of all islet genes (unpublished observations), many of which need to be examined as plausible candidates for the HNF4A-deficient hypersecretory phenotype. It is tempting to hypothesize that the initial defect that causes β-cell hypersecretion might eventually lead to β-cell exhaustion and diabetes, in analogy to what is observed in some patients with SUR1 mutations as described above [8,35,36]. However, the broad transcriptional phenotype of Hnf4a-deficient mice offers an alternative potential explanation, whereby one HNF-4α-dependent gene-expression defect causes hypersecretion early in life, while a separate gene-expression defect is responsible for the development of severe β-cell failure several years after birth.
The birthweight and incidence of hypoglycaemia in heterozygous HNF1A-mutation carriers were not different from their unaffected family members. This finding suggests that foetal insulin secretion is not increased in HNF1A-mutation carriers. Previous data had supported a common phenotype of HNF1A- and HNF4A-mutation carriers, due to a regulatory transcription factor circuit in the β-cell with positive feedback on expression between HNF-1α and HNF-4α [18,22,39,40]. Our findings suggest that, at least in foetal life, there are clearly independent functions of these two transcription factors in the foetal β-cell. Our data therefore suggest that, in humans, the proposed transcription-factor network is not critically required in foetal development and early post-natal life.
To conclude, we have shown that heterozygous HNF4A mutations are associated with a 790 g increase in birthweight, on average, and considerable risk of macrosomia. The increased birthweight is probably mediated by increased foetal insulin secretion and, in some cases, is associated with transient neonatal hyperinsulinaemia. Because HNF4A deficiency is also known to cause hypoinsulinaemic diabetes, this study shows for the first time that HNF-4α has dual opposing roles in the β-cell during different periods of life. This study also has important implications for clinical practice (see Box 1). Firstly, pregnancies where a parent is known to have an HNF4A mutation should be monitored closely during pregnancy and the immediate post-natal period to minimize complications of macrosomia and neonatal hypoglycaemia. Secondly, neonates with transient or persistent hypoglycaemia and/or macrosomia and a family history of young-onset diabetes should be considered for HNF4A molecular genetic testing. Thirdly, since the foetal genotype has a considerable impact on determining birthweight, in addition to maternal factors, paternal factors (including history of diabetes) should be considered when assessing macrosomia risk.
Box 1. Practical Clinical Points for Diagnosis and Management of Patients with HNF4A Mutations
Management of pregnancy in families known to have diabetes due to HNF4A mutations
Serial antenatal scans should be performed in any pregnancy in which the father or mother is a mutation carrier and early induction of labour is considered. This is true when the mother does not have diabetes, but particularly applies when the mother has diabetes or impaired glucose tolerance in pregnancy.
All offspring of pregnancies where the father or mother is an HNF4A-mutation carrier should be tested for neonatal hypoglycaemia at birth and also 24 h after birth.
Diagnosing MODY
In families where there is autosomal dominant inheritance of young-onset diabetes with features consistent with a diagnosis of MODY, details of birthweight and neonatal hypoglycaemia should be specifically asked for.
When macrosomia or neonatal hypoglycaemia (>24 h) is described, HNF4A should be sequenced before other genes when performing diagnostic genetic testing.
Diagnosing and managing neonatal hypoglycaemia
HNF4A should be sequenced in children with neonatal hypoglycaemia, particularly if the hypoglycaemia is relatively mild or transient, or if a family member (parent or grandparent) has young-onset diabetes (<35 y).
In patients diagnosed as having HNF4A, resolution of symptoms should be expected in the first year, but diabetes should be expected to develop in adolescence or in early adulthood and should be screened for annually after the age of 10 y.
Investigating macrosomia
HNF4A should be sequenced as part of an investigation of unexplained macrosomia, particularly when the macrosomia is extreme, is accompanied by hypoglycaemia, or there is a family history of early-onset diabetes.
Supporting Information
(29 KB DOC)
Data are mean ± standard error of the mean from three different control and mutant mice, except for Kcnj11 where six control and six mutant mice were analysed.
(28 KB PPT)
Accession Numbers
The GenBank (http://www.ncbi.nlm.nih.gov/Genbank) identification numbers for the genes and gene products discussed in this paper are HNF1A/TCF1 (NM_000545.3) and HNF4A (NM_000457.3).
Acknowledgments
The authors would like to acknowledge the patients and their referring clinicians, especially Kathryn Noyes and the genetic diabetes nurses Linda Robertson Jacqueline Jones, and Helen John, as well as Natalia del Pozo for technical assistance. Pedro L. Herrera (University of Geneva) is thanked for InsPr-Cre mice. ERP is a Clinician Scientist Fellow (National Health Service Education for Scotland), and ATH is a Wellcome Trust Research Leave Fellow.
Abbreviation
- MODY
maturity-onset diabetes of the young
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Author contributions. ERP, SFB, AMS, SE, JF, and ATH were involved with the study design and data analysis. The mouse studies were undertaken by SFB and JF. The human molecular genetic analysis was carried out by KS and SE. All authors were involved with interpretation of the data and preparation of the manuscript.
Funding: This study was funded by the Wellcome Trust, National Health Service Education for Scotland, National Health Service Research and Development, Eurodia (EC 6th Framework Programme), and Instituto de Salud Carlos III. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- American College of Obstetricians and Gynecologists (ACOG) ACOG Practice Bulletin no. 22. Washington (DC): American College of Obstetricians and Gynecologists (ACOG); 2000. Fetal macrosomia. 11 November 2000. [Google Scholar]
- Chauhan SP, Grobman WA, Gherman RA, Chauhan VB, Chang G, et al. Suspicion and treatment of the macrosomic fetus: A review. Am J Obstet Gynecol. 2005;193:332–346. doi: 10.1016/j.ajog.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Thomas PM, Cote GJ, Wohllk N, Haddad B, Mathew PM, et al. Mutations in the sulfonylurea receptor gene in familial persistent hyperinsulinemic hypoglycemia of infancy. Science. 1995;268:426–429. doi: 10.1126/science.7716548. [DOI] [PubMed] [Google Scholar]
- Thomas P, Ye Y, Lightner E. Mutation of the pancreatic islet inward rectifier Kir6.2 also leads to familial persistent hyperinsulinemic hypoglycemia of infancy. Hum Mol Genet. 1996;5:1809–1812. doi: 10.1093/hmg/5.11.1809. [DOI] [PubMed] [Google Scholar]
- Dunne MJ, Kane C, Shepherd RM, Sanchez JA, James RF, et al. Familial persistent hyperinsulinemic hypoglycemia of infancy and mutations in the sulfonylurea receptor. N Engl J Med. 1997;336:703–706. doi: 10.1056/NEJM199703063361005. [DOI] [PubMed] [Google Scholar]
- Glaser B, Kesavan P, Heyman M, Davis E, Cuesta A, et al. Familial hyperinsulinism caused by an activating glucokinase mutation. N Engl J Med. 1998;338:226–230. doi: 10.1056/NEJM199801223380404. [DOI] [PubMed] [Google Scholar]
- Stanley CA, Lieu YK, Hsu BY, Burlina AB, Greenberg CR, et al. Hyperinsulinism and hyperammonemia in infants with regulatory mutations of the glutamate dehydrogenase gene. N Engl J Med. 1998;338:1352–1357. doi: 10.1056/NEJM199805073381904. [DOI] [PubMed] [Google Scholar]
- Huopio H, Reimann F, Ashfield R, Komulainen J, Lenko HL, et al. Dominantly inherited hyperinsulinism caused by a mutation in the sulfonylurea receptor type 1. J Clin Invest. 2000;106:897–906. doi: 10.1172/JCI9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton PT, Eaton S, Aynsley-Green A, Edginton M, Hussain K, et al. Hyperinsulinism in short-chain L-3-hydroxyacyl-CoA dehydrogenase deficiency reveals the importance of beta-oxidation in insulin secretion. J Clin Invest. 2001;108:457–465. doi: 10.1172/JCI11294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molven A, Matre GE, Duran M, Wanders RJ, Rishaug U, et al. Familial hyperinsulinemic hypoglycemia caused by a defect in the SCHAD enzyme of mitochondrial fatty acid oxidation. Diabetes. 2004;53:221–227. doi: 10.2337/diabetes.53.1.221. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Cummings EA, Edghill EL, Harries LW, Scott R, et al. Permanent neonatal diabetes due to paternal germline mosaicism for an activating mutation of the KCNJ11 gene encoding the Kir6.2 subunit of the beta-cell potassium adenosine triphosphate channel. J Clin Endocrinol Metab. 2004;89:3932–3935. doi: 10.1210/jc.2004-0568. [DOI] [PubMed] [Google Scholar]
- Proks P, Arnold AL, Bruining J, Girard C, Flanagan SE, et al. A heterozygous activating mutation in the sulphonylurea receptor SUR1 (ABCC8) causes neonatal diabetes. Hum Mol Genet. 2006;15:1793–1800. doi: 10.1093/hmg/ddl101. [DOI] [PubMed] [Google Scholar]
- Hattersley AT, Beards F, Ballantyne E, Appleton M, Harvey R, et al. Mutations in the glucokinase gene of the fetus result in reduced birth weight. Nat Genet. 1998;19:268–270. doi: 10.1038/953. [DOI] [PubMed] [Google Scholar]
- Edghill EL, Bingham C, Slingerland AS, Minton JA, Noordam C, et al. Hepatocyte nuclear factor-1 beta mutations cause neonatal diabetes and intrauterine growth retardation: Support for a critical role of HNF-1beta in human pancreatic development. Diabet Med. 2006;23:1301–1306. doi: 10.1111/j.1464-5491.2006.01999.x. [DOI] [PubMed] [Google Scholar]
- Byrne MM, Sturis J, Fajans SS, Ortiz FJ, Stoltz A, et al. Altered insulin secretory responses to glucose in subjects with a mutation in the MODY1 gene on chromosome 20. Diabetes. 1995;44:699–704. doi: 10.2337/diab.44.6.699. [DOI] [PubMed] [Google Scholar]
- Byrne MM, Sturis J, Menzel S, Yamagata K, Fajans SS, et al. Altered insulin secretory responses to glucose in diabetic and nondiabetic subjects with mutations in the diabetes susceptibility gene MODY3 on Chromosome 12. Diabetes. 1996;45:1503–1510. doi: 10.2337/diab.45.11.1503. [DOI] [PubMed] [Google Scholar]
- Odom DT, Zizlsperger N, Gordon DB, Bell GW, Rinaldi NJ, et al. Control of pancreas and liver gene expression by HNF transcription factors. Science. 2004;303:1378–1381. doi: 10.1126/science.1089769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer J. A genetic switch in pancreatic beta-cells: Implications for differentiation and haploinsufficiency. Diabetes. 2002;51:2355–2362. doi: 10.2337/diabetes.51.8.2355. [DOI] [PubMed] [Google Scholar]
- Silander K, Mohlke KL, Scott LJ, Peck EC, Hollstein P, et al. Genetic variation near the hepatocyte nuclear factor-4 alpha gene predicts susceptibility to type 2 diabetes. Diabetes. 2004;53:1141–1149. doi: 10.2337/diabetes.53.4.1141. [DOI] [PubMed] [Google Scholar]
- Love-Gregory LD, Wasson J, Ma J, Jin CH, Glaser B, et al. A common polymorphism in the upstream promoter region of the hepatocyte nuclear factor-4 alpha gene on chromosome 20q is associated with type 2 diabetes and appears to contribute to the evidence for linkage in an Ashkenazi Jewish population. Diabetes. 2004;53:1134–1140. doi: 10.2337/diabetes.53.4.1134. [DOI] [PubMed] [Google Scholar]
- Gupta RK, Vatamaniuk MZ, Lee CS, Flaschen RC, Fulmer JT, et al. The MODY1 gene HNF-4alpha regulates selected genes involved in insulin secretion. J Clin Invest. 2005;115:1006–1015. doi: 10.1172/JCI200522365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson ER, Pruhova S, Tack CJ, Johansen A, Castleden HA, et al. Molecular genetics and phenotypic characteristics of MODY caused by hepatocyte nuclear factor 4alpha mutations in a large European collection. Diabetologia. 2005;48:878–885. doi: 10.1007/s00125-005-1738-y. [DOI] [PubMed] [Google Scholar]
- Freeman JV, Cole TJ, Chinn S, Jones PR, White EM, et al. Cross sectional stature and weight reference curves for the UK, 1990. Arch Dis Child. 1995;73:17–24. doi: 10.1136/adc.73.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayhurst GP, Lee YH, Lambert G, Ward JM, Gonzalez FJ. Hepatocyte nuclear factor 4alpha (nuclear receptor 2A1) is essential for maintenance of hepatic gene expression and lipid homeostasis. Mol Cell Biol. 2001;21:1393–1403. doi: 10.1128/MCB.21.4.1393-1403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- Luco RF, Maestro MA, Del Pozo N, Philbrick WM, de la Ossa PP, et al. A conditional model reveals that induction of hepatocyte nuclear factor-1alpha in hnf1alpha-null mutant beta-cells can activate silenced genes postnatally, whereas overexpression is deleterious. Diabetes. 2006;55:2202–2211. doi: 10.2337/db05-1534. [DOI] [PubMed] [Google Scholar]
- Boulet SL, Alexander GR, Salihu HM, Pass M. Macrosomic births in the United States: Determinants, outcomes, and proposed grades of risk. Am J Obstet Gynecol. 2003;188:1372–1378. doi: 10.1067/mob.2003.302. [DOI] [PubMed] [Google Scholar]
- Accili D, Drago J, Lee EJ, Johnson MD, Cool MH, et al. Early neonatal death in mice homozygous for a null allele of the insulin receptor gene. Nat Genet. 1996;12:106–109. doi: 10.1038/ng0196-106. [DOI] [PubMed] [Google Scholar]
- Stoffel M, Duncan SA. The maturity-onset diabetes of the young (MODY1) transcription factor HNF4alpha regulates expression of genes required for glucose transport and metabolism. Proc Natl Acad Sci U S A. 1997;94:13209–13214. doi: 10.1073/pnas.94.24.13209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Maechler P, Antinozzi PA, Hagenfeldt KA, Wollheim CB. Hepatocyte nuclear factor 4alpha regulates the expression of pancreatic beta-cell genes implicated in glucose metabolism and nutrient-induced insulin secretion. J Biol Chem. 2000;275:35953–35959. doi: 10.1074/jbc.M006612200. [DOI] [PubMed] [Google Scholar]
- Stoffers DA, Zinkin NT, Stanojevic V, Clarke WL, Habener JF. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Slingerland AS, Hattersley AT. Activating mutations in the gene encoding Kir6.2 alter fetal and postnatal growth as well as causing neonatal diabetes. J Clin Endocrinol Metab. 2006;91:2782–2788. doi: 10.1210/jc.2006-0201. [DOI] [PubMed] [Google Scholar]
- Babenko AP, Polak M, Cave H, Busiah K, Czernichow P, et al. Activating mutations in the ABCC8 gene in neonatal diabetes mellitus. N Engl J Med. 2006;355:456–466. doi: 10.1056/NEJMoa055068. [DOI] [PubMed] [Google Scholar]
- Leibowitz G, Glaser B, Higazi AA, Salameh M, Cerasi E, et al. Hyperinsulinemic hypoglycemia of infancy (nesidioblastosis) in clinical remission: High incidence of diabetes mellitus and persistent beta-cell dysfunction at long-term follow-up. J Clin Endocrinol Metab. 1995;80:386–392. doi: 10.1210/jcem.80.2.7852494. [DOI] [PubMed] [Google Scholar]
- Huopio H, Otonkoski T, Vauhkonen I, Reimann F, Ashcroft FM, et al. A new subtype of autosomal dominant diabetes attributable to a mutation in the gene for sulfonylurea receptor 1. Lancet. 2003;361:301–307. doi: 10.1016/S0140-6736(03)12325-2. [DOI] [PubMed] [Google Scholar]
- Kassem SA, Ariel I, Thornton PS, Scheimberg I, Glaser B. Beta-cell proliferation and apoptosis in the developing normal human pancreas and in hyperinsulinism of infancy. Diabetes. 2000;49:1325–1333. doi: 10.2337/diabetes.49.8.1325. [DOI] [PubMed] [Google Scholar]
- Miura A, Yamagata K, Kakei M, Hatakeyama H, Takahashi N, et al. Hepatocyte nuclear factor-4alpha is essential for glucose-stimulated insulin secretion by pancreatic beta-cells. J Biol Chem. 2006;281:5246–5257. doi: 10.1074/jbc.M507496200. [DOI] [PubMed] [Google Scholar]
- Thomas H, Jaschkowitz K, Bulman M, Frayling TM, Mitchell SM, et al. A distant upstream promoter of the HNF-4alpha gene connects the transcription factors involved in maturity-onset diabetes of the young. Hum Mol Genet. 2001;10:2089–2097. doi: 10.1093/hmg/10.19.2089. [DOI] [PubMed] [Google Scholar]
- Boj SF, Parrizas M, Maestro MA, Ferrer J. A transcription factor regulatory circuit in differentiated pancreatic cells. Proc Natl Acad Sci U S A. 2001;98:14481–14486. doi: 10.1073/pnas.241349398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(29 KB DOC)
Data are mean ± standard error of the mean from three different control and mutant mice, except for Kcnj11 where six control and six mutant mice were analysed.
(28 KB PPT)