Abstract
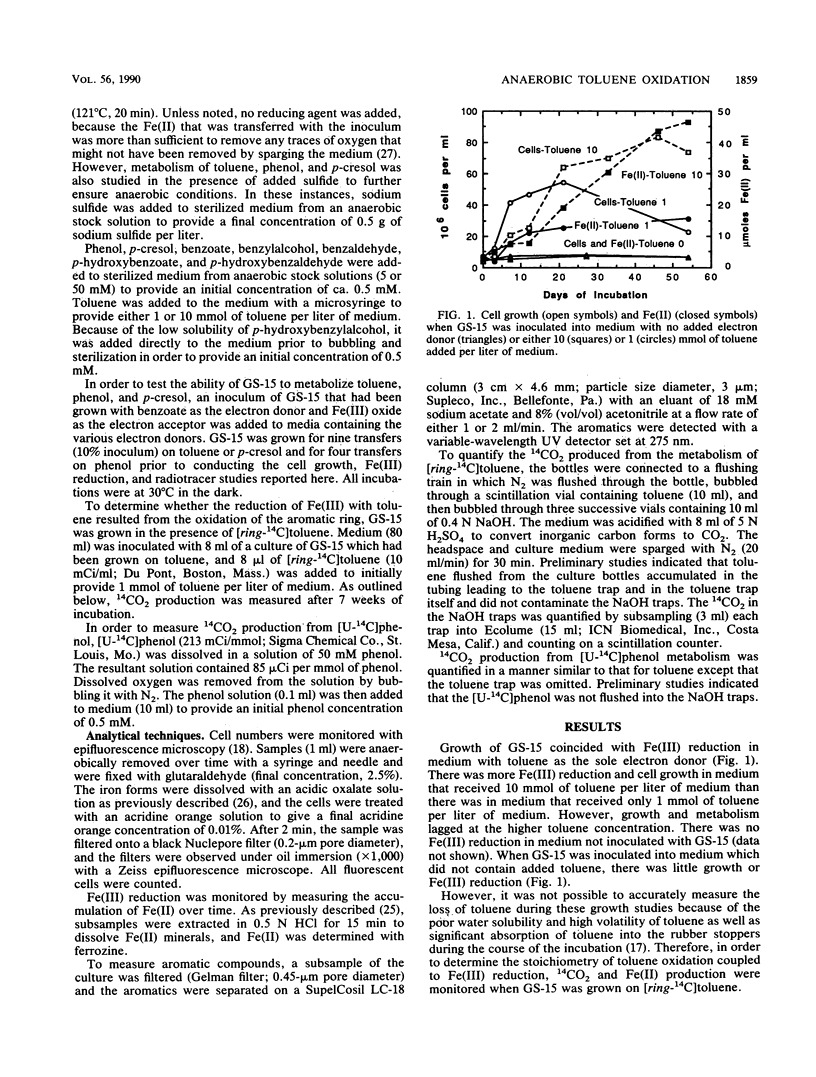
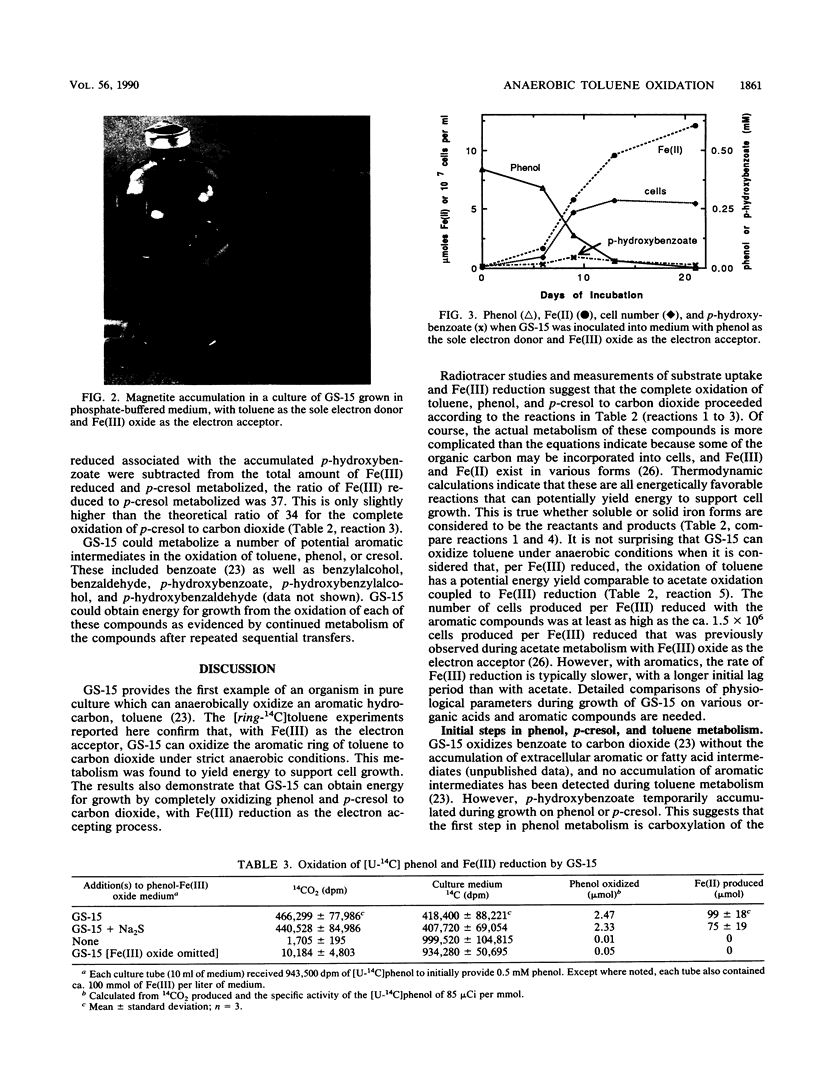
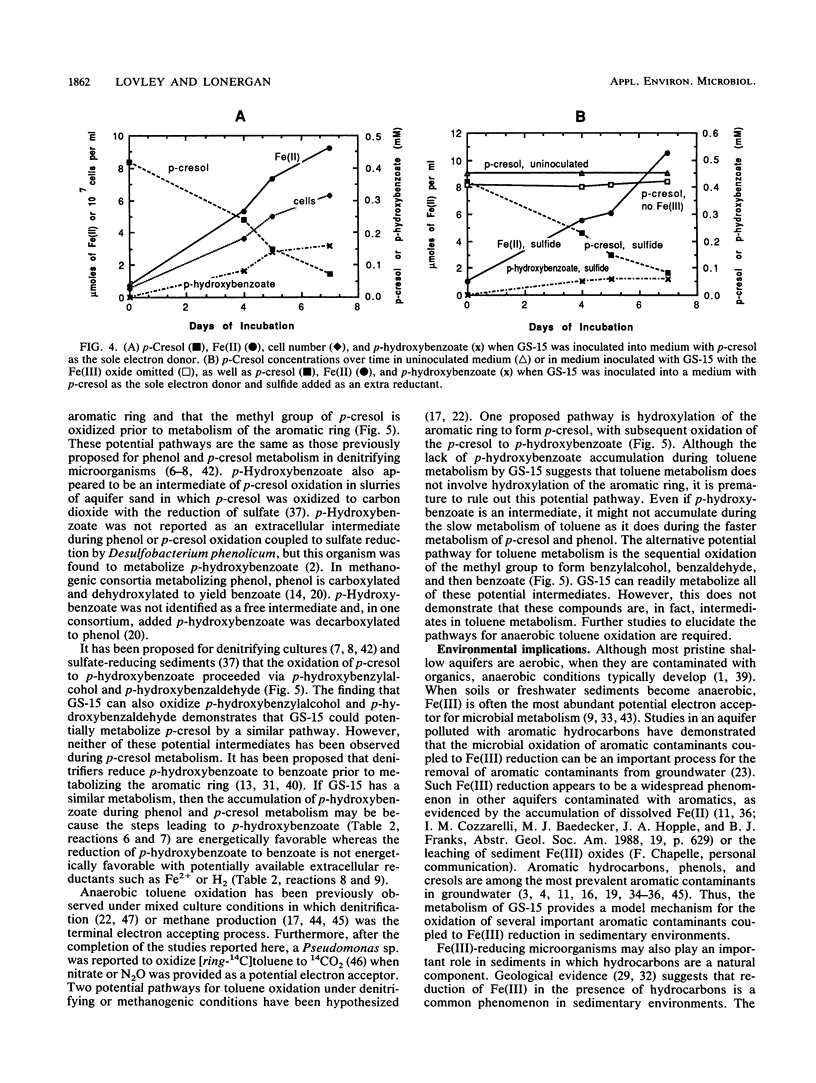
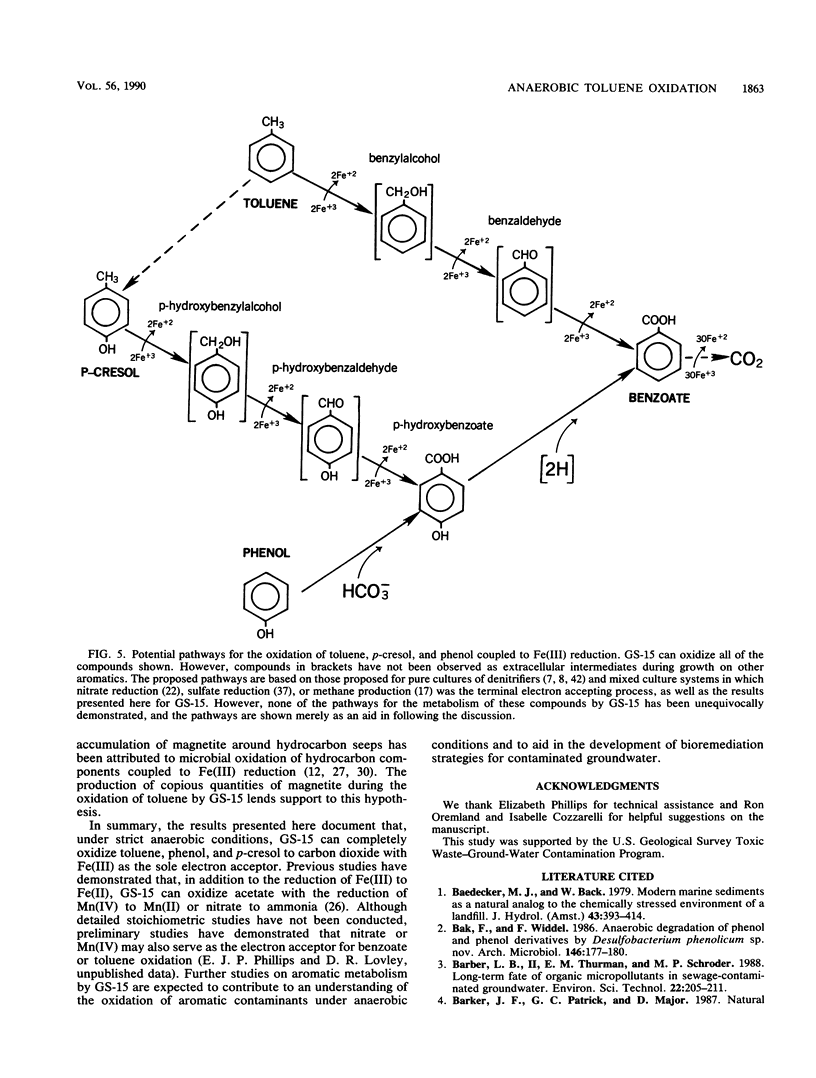
The dissimilatory Fe(III) reducer, GS-15, is the first microorganism known to couple the oxidation of aromatic compounds to the reduction of Fe(III) and the first example of a pure culture of any kind known to anaerobically oxidize an aromatic hydrocarbon, toluene. In this study, the metabolism of toluene, phenol, and p-cresol by GS-15 was investigated in more detail. GS-15 grew in an anaerobic medium with toluene as the sole electron donor and Fe(III) oxide as the electron acceptor. Growth coincided with Fe(III) reduction. [ring-14C]toluene was oxidized to 14CO2, and the stoichiometry of 14CO2 production and Fe(III) reduction indicated that GS-15 completely oxidized toluene to carbon dioxide with Fe(III) as the electron acceptor. Magnetite was the primary iron end product during toluene oxidation. Phenol and p-cresol were also completely oxidized to carbon dioxide with Fe(III) as the sole electron acceptor, and GS-15 could obtain energy to support growth by oxidizing either of these compounds as the sole electron donor. p-Hydroxybenzoate was a transitory extracellular intermediate of phenol and p-cresol metabolism but not of toluene metabolism. GS-15 oxidized potential aromatic intermediates in the oxidation of toluene (benzylalcohol and benzaldehyde) and p-cresol (p-hydroxybenzylalcohol and p-hydroxybenzaldehyde). The metabolism described here provides a model for how aromatic hydrocarbons and phenols may be oxidized with the reduction of Fe(III) in contaminated aquifers and petroleum-containing sediments.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berry D. F., Francis A. J., Bollag J. M. Microbial metabolism of homocyclic and heterocyclic aromatic compounds under anaerobic conditions. Microbiol Rev. 1987 Mar;51(1):43–59. doi: 10.1128/mr.51.1.43-59.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert I. D., Whited G., Gibson D. T., Young L. Y. Anaerobic oxidation of p-cresol mediated by a partially purified methylhydroxylase from a denitrifying bacterium. J Bacteriol. 1989 Jun;171(6):2956–2962. doi: 10.1128/jb.171.6.2956-2962.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossert I. D., Young L. Y. Anaerobic oxidation of p-cresol by a denitrifying bacterium. Appl Environ Microbiol. 1986 Nov;52(5):1117–1122. doi: 10.1128/aem.52.5.1117-1122.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. C., Fuchs G. Anaerobic degradation of aromatic compounds. Annu Rev Microbiol. 1988;42:289–317. doi: 10.1146/annurev.mi.42.100188.001445. [DOI] [PubMed] [Google Scholar]
- Genthner B. R., Townsend G. T., Chapman P. J. Anaerobic transformation of phenol to benzoate via para-carboxylation: use of fluorinated analogues to elucidate the mechanism of transformation. Biochem Biophys Res Commun. 1989 Aug 15;162(3):945–951. doi: 10.1016/0006-291x(89)90764-x. [DOI] [PubMed] [Google Scholar]
- Grbić-Galić D., Vogel T. M. Transformation of toluene and benzene by mixed methanogenic cultures. Appl Environ Microbiol. 1987 Feb;53(2):254–260. doi: 10.1128/aem.53.2.254-260.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie J. E., Daley R. J., Jasper S. Use of nuclepore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977 May;33(5):1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn E. P., Zeyer J., Eicher P., Schwarzenbach R. P. Anaerobic degradation of alkylated benzenes in denitrifying laboratory aquifer columns. Appl Environ Microbiol. 1988 Feb;54(2):490–496. doi: 10.1128/aem.54.2.490-496.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Availability of ferric iron for microbial reduction in bottom sediments of the freshwater tidal potomac river. Appl Environ Microbiol. 1986 Oct;52(4):751–757. doi: 10.1128/aem.52.4.751-757.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Novel mode of microbial energy metabolism: organic carbon oxidation coupled to dissimilatory reduction of iron or manganese. Appl Environ Microbiol. 1988 Jun;54(6):1472–1480. doi: 10.1128/aem.54.6.1472-1480.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Organic matter mineralization with reduction of ferric iron in anaerobic sediments. Appl Environ Microbiol. 1986 Apr;51(4):683–689. doi: 10.1128/aem.51.4.683-689.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa T., Maruyama Y. Anaerobic metabolism of phthalate and other aromatic compounds by a denitrifying bacterium. J Bacteriol. 1988 Dec;170(12):5778–5784. doi: 10.1128/jb.170.12.5778-5784.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolenski W. J., Suflita J. M. Biodegradation of cresol isomers in anoxic aquifers. Appl Environ Microbiol. 1987 Apr;53(4):710–716. doi: 10.1128/aem.53.4.710-716.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. F., Campbell W. L., Chinoy I. Anaerobic degradation of the benzene nucleus by a facultatively anaerobic microorganism. J Bacteriol. 1970 May;102(2):430–437. doi: 10.1128/jb.102.2.430-437.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thauer R. K., Jungermann K., Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977 Mar;41(1):100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tschech A., Fuchs G. Anaerobic degradation of phenol by pure cultures of newly isolated denitrifying pseudomonads. Arch Microbiol. 1987 Sep;148(3):213–217. doi: 10.1007/BF00414814. [DOI] [PubMed] [Google Scholar]
- Vogel T. M., Grbìc-Galìc D. Incorporation of Oxygen from Water into Toluene and Benzene during Anaerobic Fermentative Transformation. Appl Environ Microbiol. 1986 Jul;52(1):200–202. doi: 10.1128/aem.52.1.200-202.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeyer J., Kuhn E. P., Schwarzenbach R. P. Rapid microbial mineralization of toluene and 1,3-dimethylbenzene in the absence of molecular oxygen. Appl Environ Microbiol. 1986 Oct;52(4):944–947. doi: 10.1128/aem.52.4.944-947.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]




