Abstract
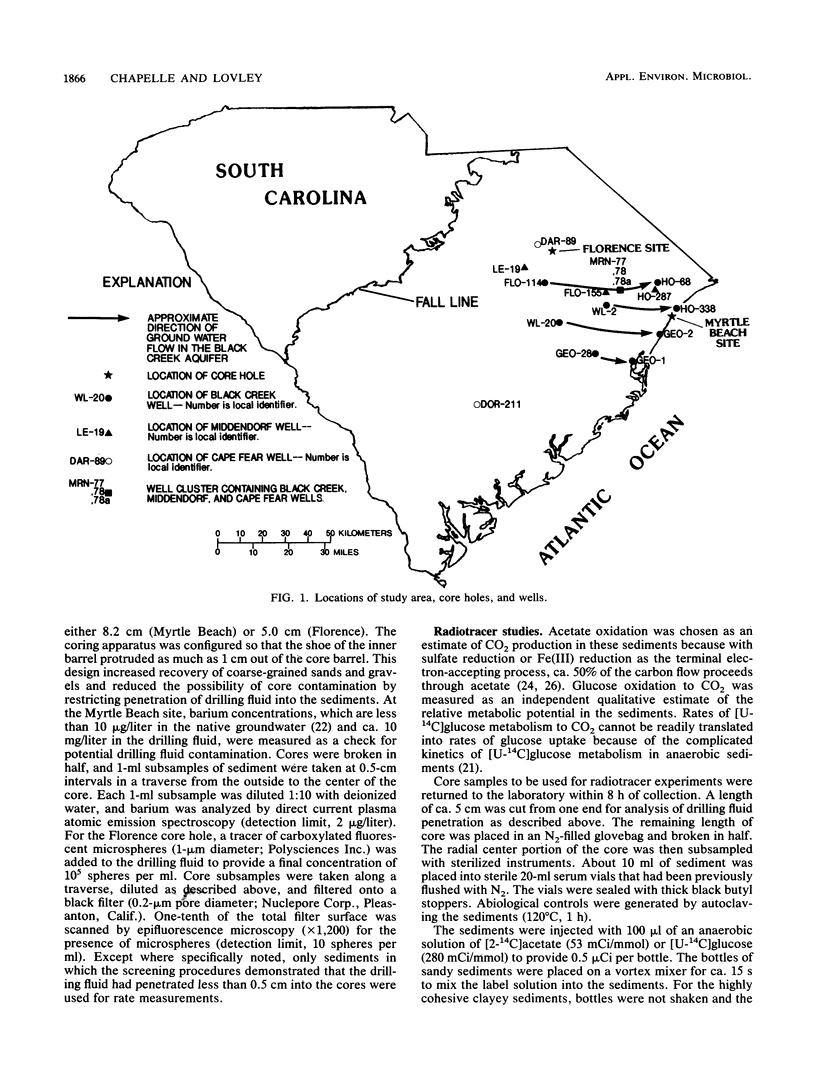
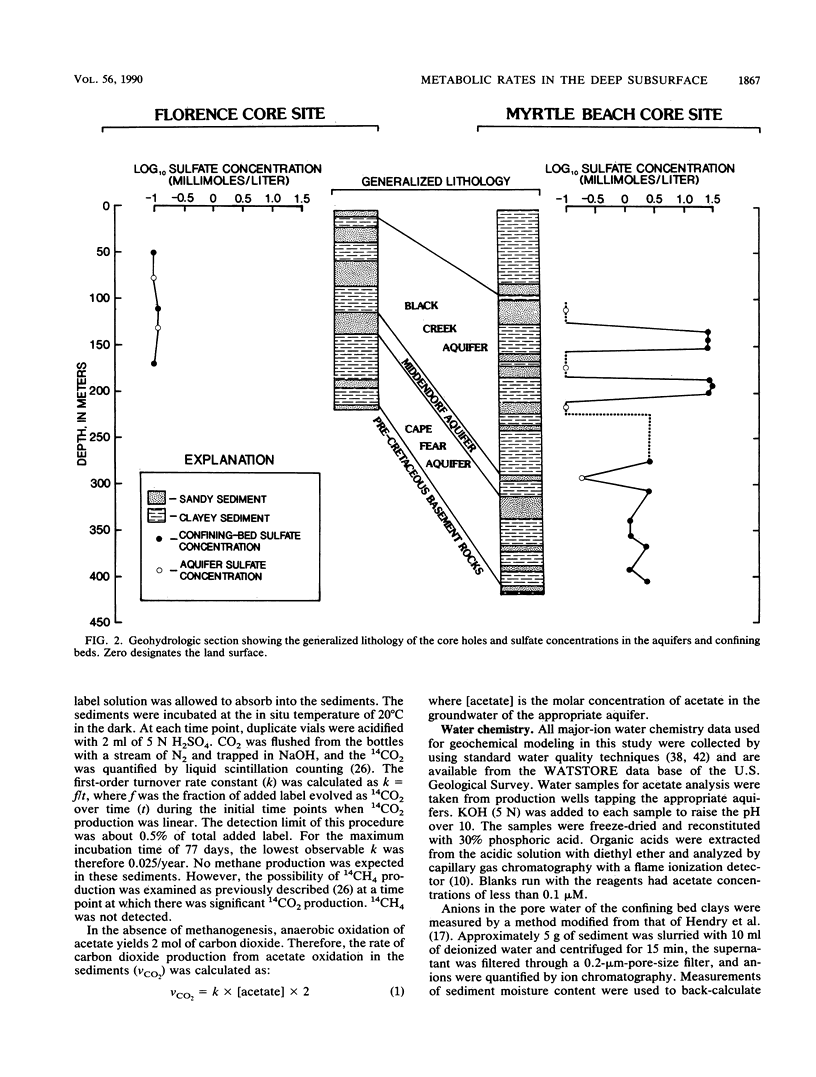
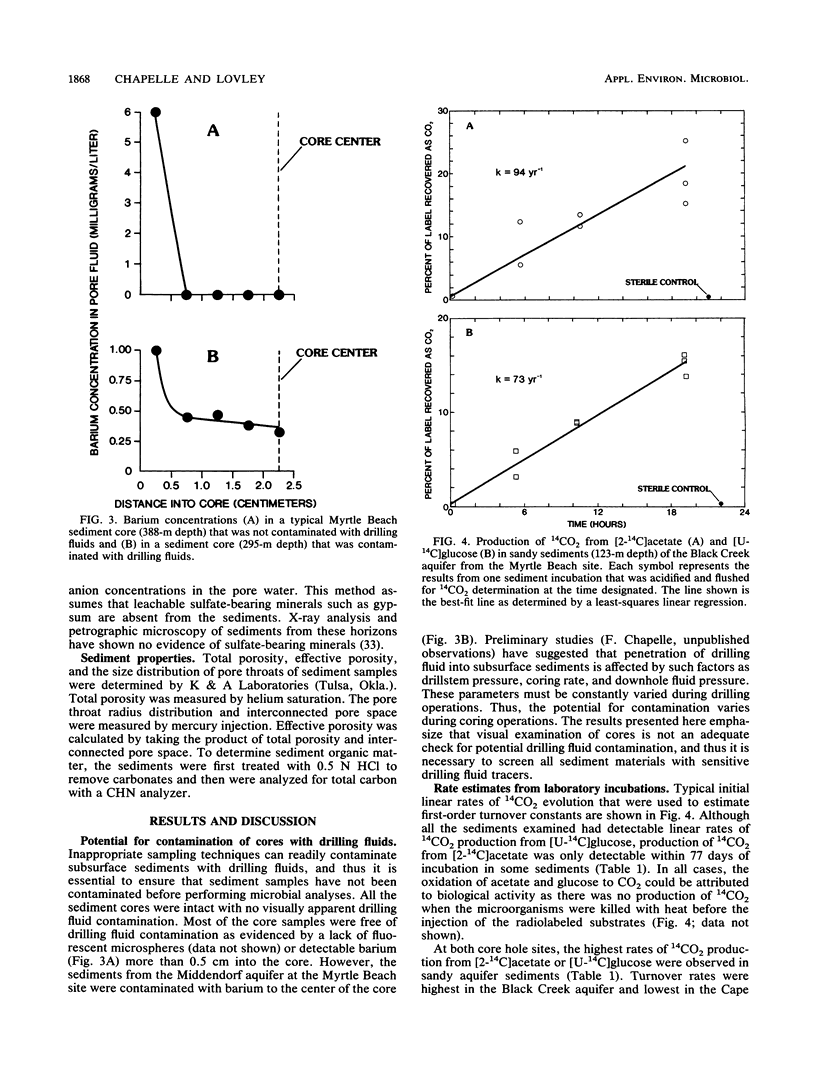
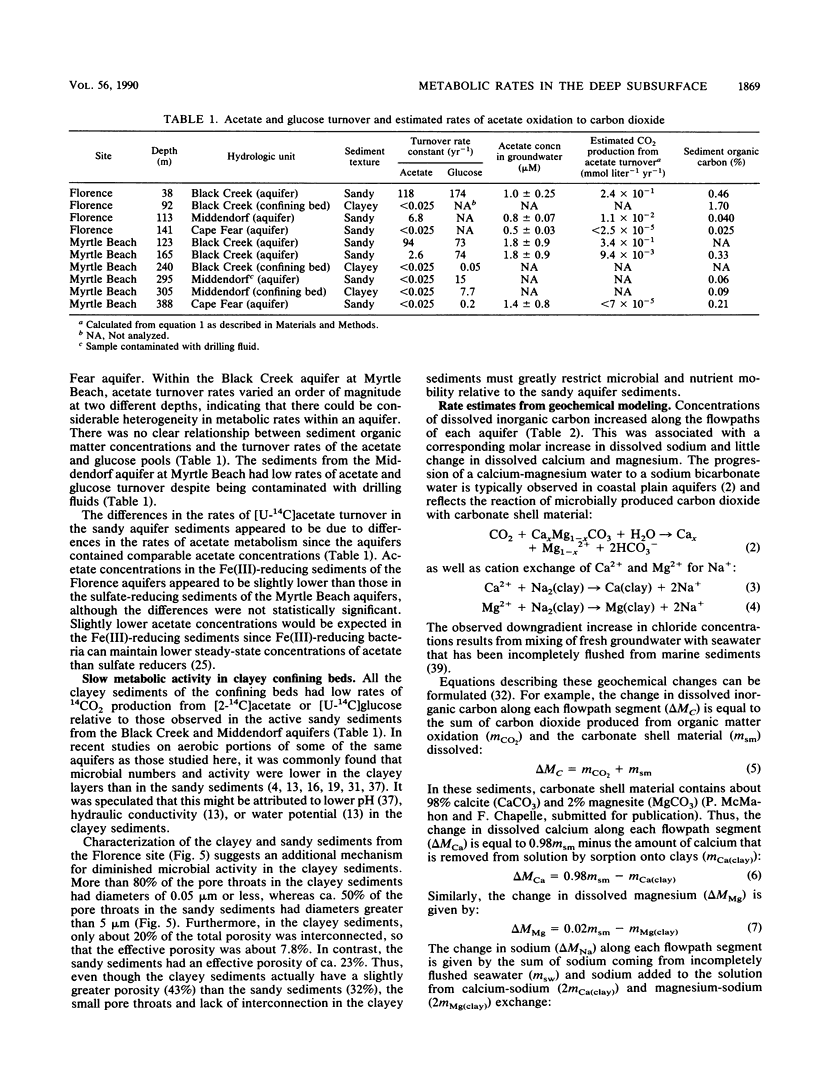
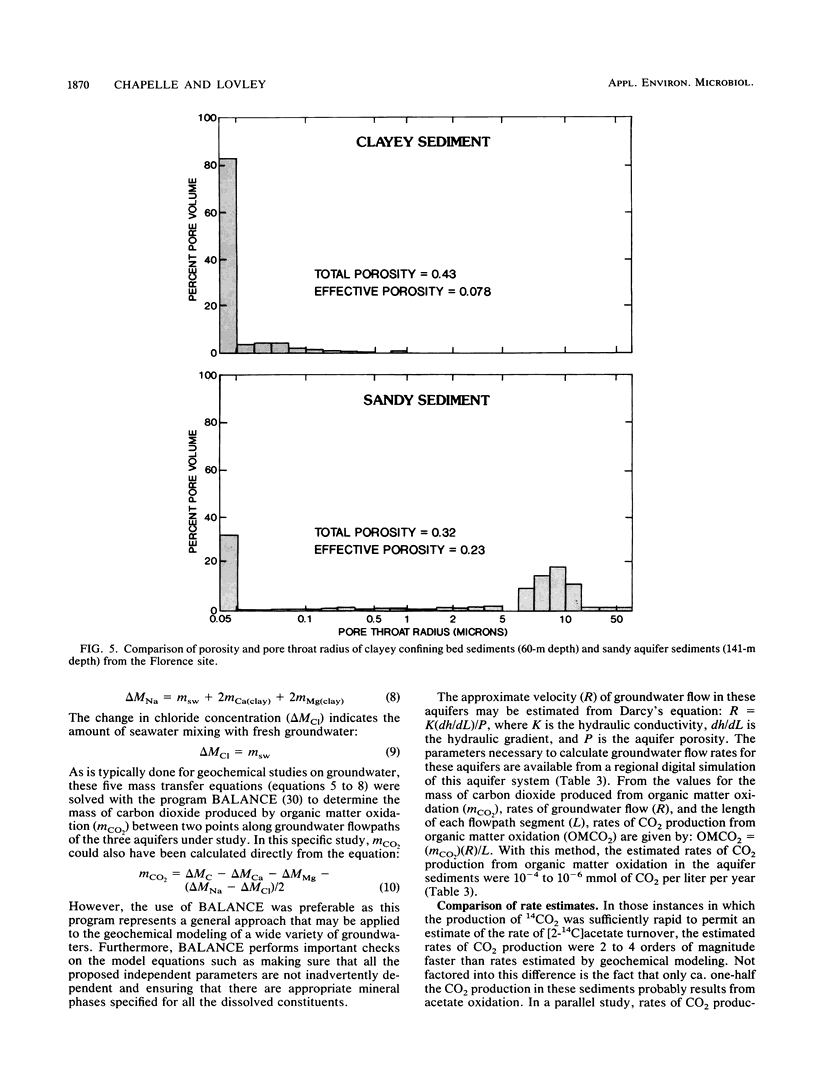
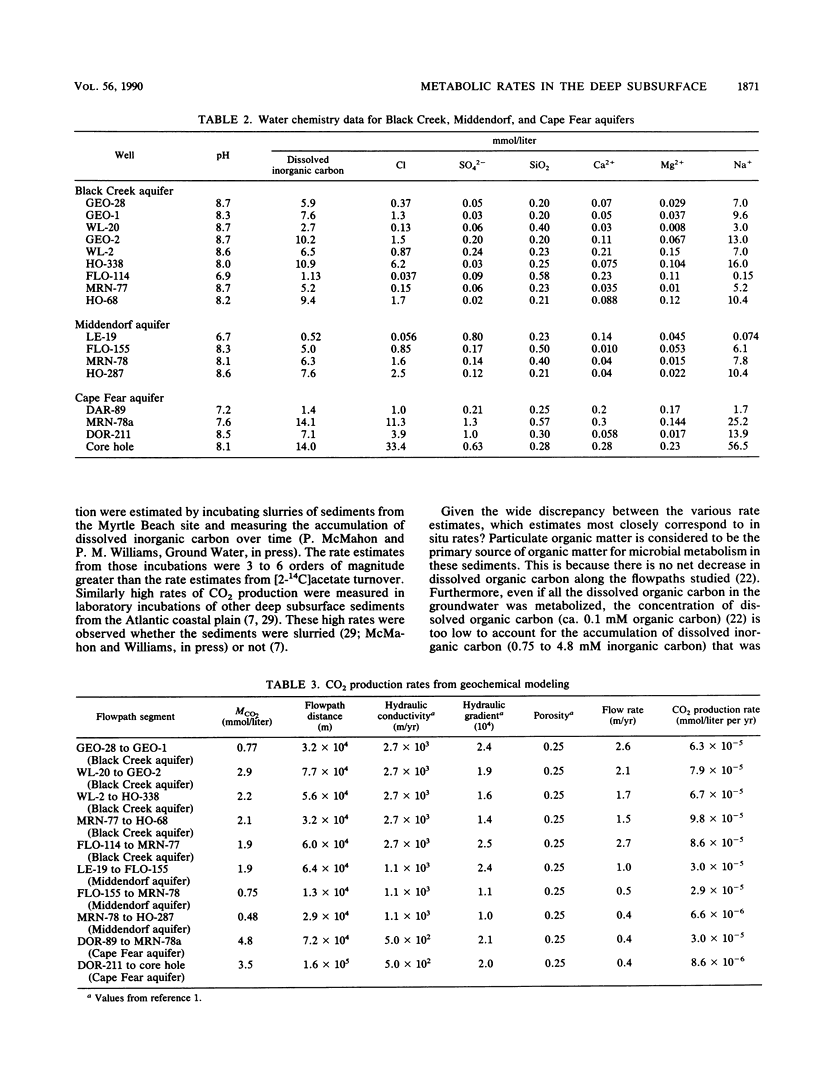
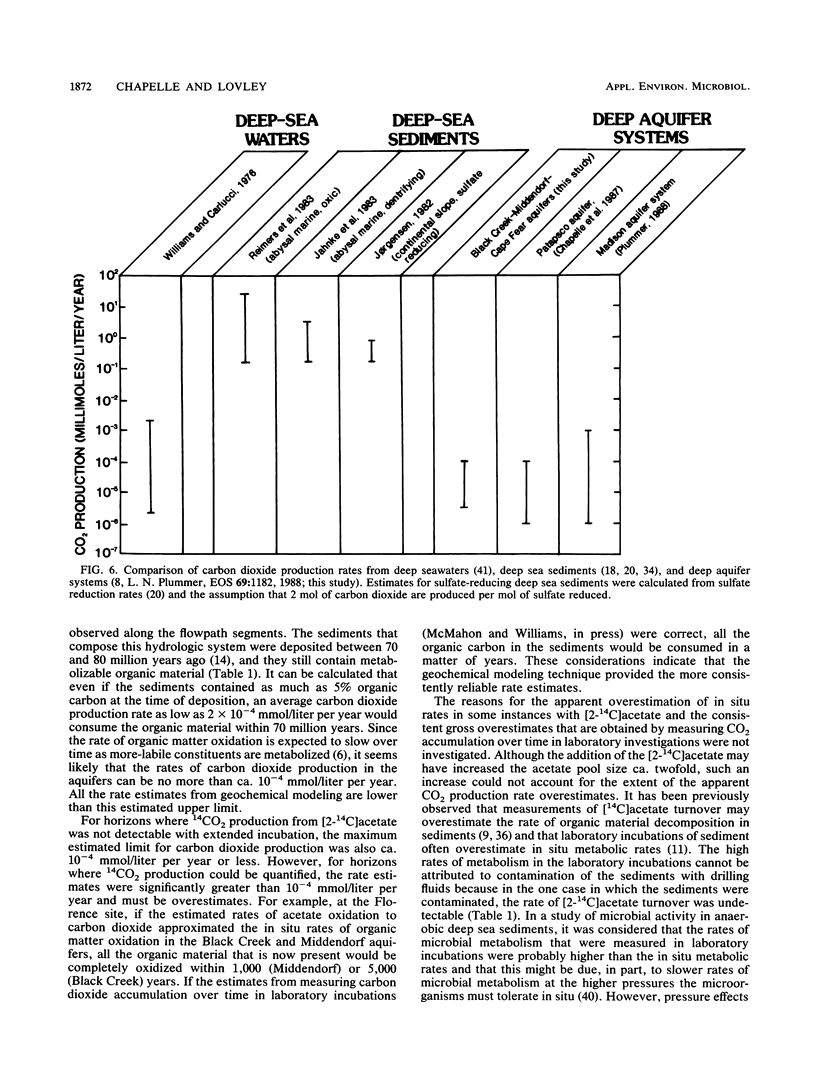
Rates of microbial metabolism in deep anaerobic aquifers of the Atlantic coastal plain of South Carolina were investigated by both microbiological and geochemical techniques. Rates of [2-14C]acetate and [U-14C]glucose oxidation as well as geochemical evidence indicated that metabolic rates were faster in the sandy sediments composing the aquifers than in the clayey sediments of the confining layers. In the sandy aquifer sediments, estimates of the rates of CO2 production (millimoles of CO2 per liter per year) based on the oxidation of [2-14C] acetate were 9.4 × 10−3 to 2.4 × 10−1 for the Black Creek aquifer, 1.1 × 10−2 for the Middendorf aquifer, and <7 × 10−5 for the Cape Fear aquifer. These estimates were at least 2 orders of magnitude lower than previously published estimates that were based on the accumulation of CO2 in laboratory incubations of similar deep subsurface sediments. In contrast, geochemical modeling of groundwater chemistry changes along aquifer flowpaths gave rate estimates that ranged from 10−4 to 10−6 mmol of CO2 per liter per year. The age of these sediments (ca. 80 million years) and their organic carbon content suggest that average rates of CO2 production could have been no more than 10−4 mmol per liter per year. Thus, laboratory incubations may greatly overestimate the in situ rates of microbial metabolism in deep subsurface environments. This has important implications for the use of laboratory incubations in attempts to estimate biorestoration capacities of deep aquifers. The rate estimates from geochemical modeling indicate that deep aquifers are among the most oligotrophic aquatic environments in which there is ongoing microbial metabolism.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- King G. M., Klug M. J. Glucose metabolism in sediments of a eutrophic lake: tracer analysis of uptake and product formation. Appl Environ Microbiol. 1982 Dec;44(6):1308–1317. doi: 10.1128/aem.44.6.1308-1317.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Competitive mechanisms for inhibition of sulfate reduction and methane production in the zone of ferric iron reduction in sediments. Appl Environ Microbiol. 1987 Nov;53(11):2636–2641. doi: 10.1128/aem.53.11.2636-2641.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovley D. R., Phillips E. J. Requirement for a Microbial Consortium To Completely Oxidize Glucose in Fe(III)-Reducing Sediments. Appl Environ Microbiol. 1989 Dec;55(12):3234–3236. doi: 10.1128/aem.55.12.3234-3236.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams P. M., Carlucci A. F. Bacterial utilisation of organic matter in the deep sea. Nature. 1976 Aug 26;262(5571):810–811. doi: 10.1038/262810a0. [DOI] [PubMed] [Google Scholar]


