Abstract
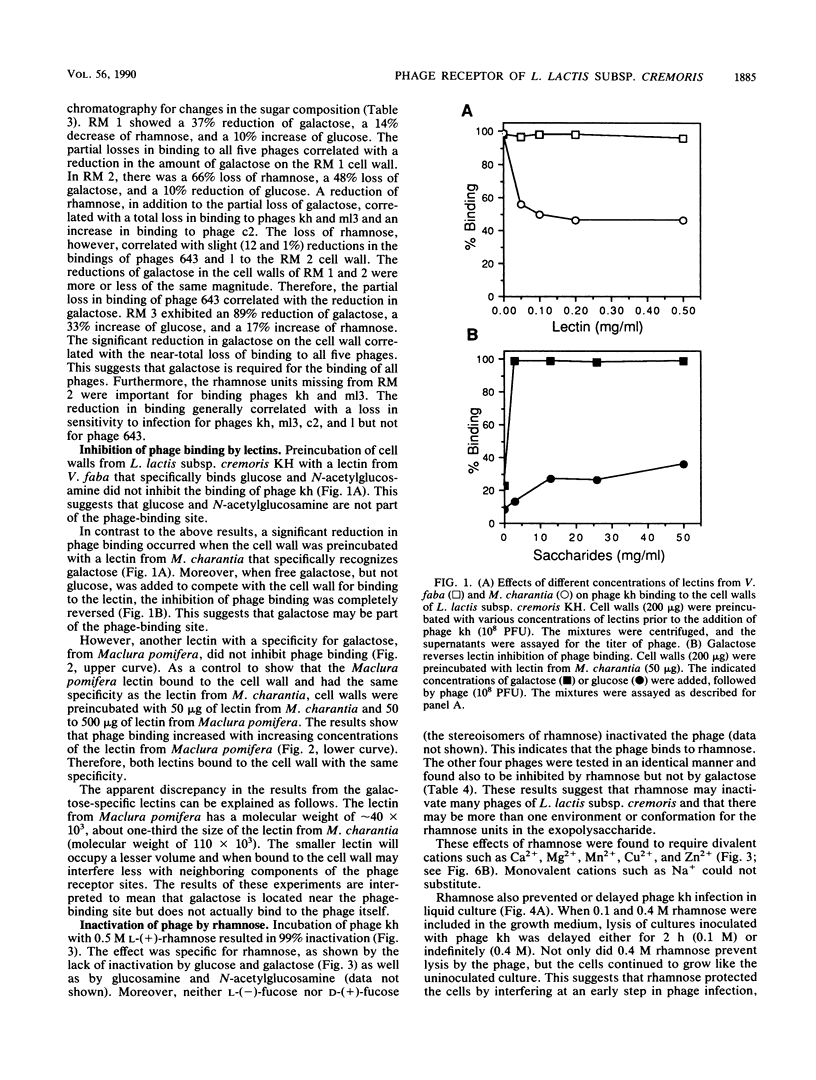
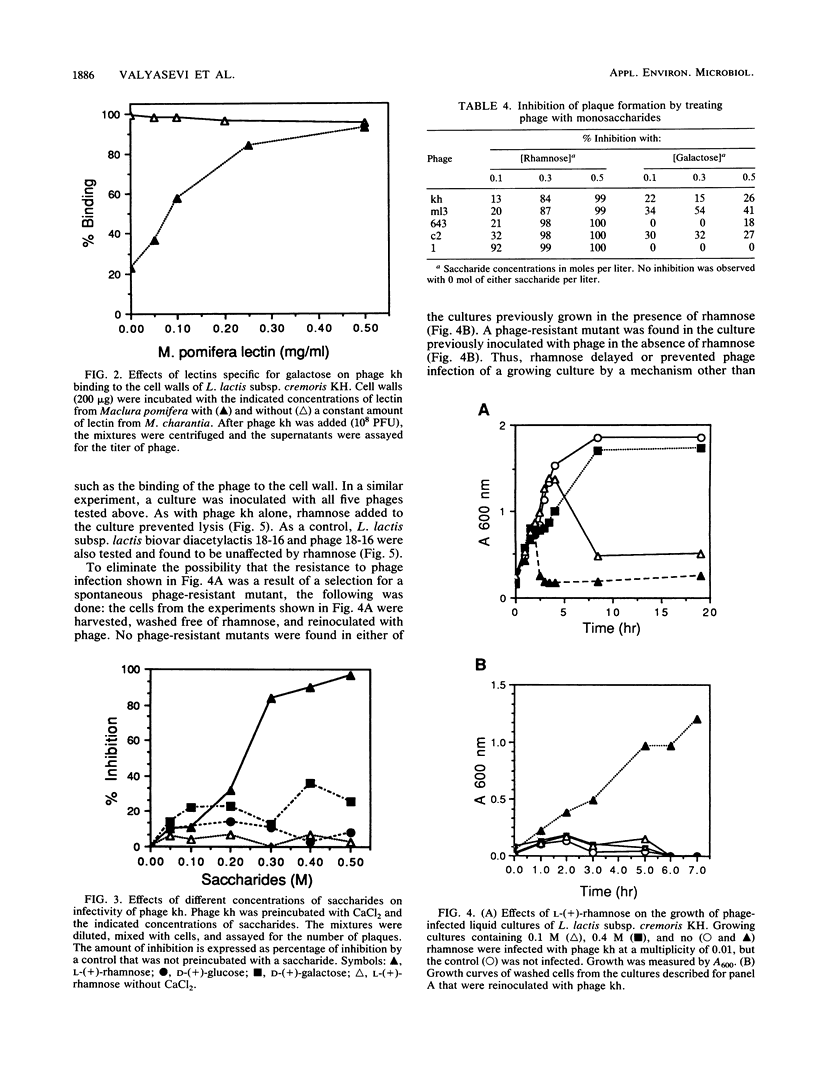
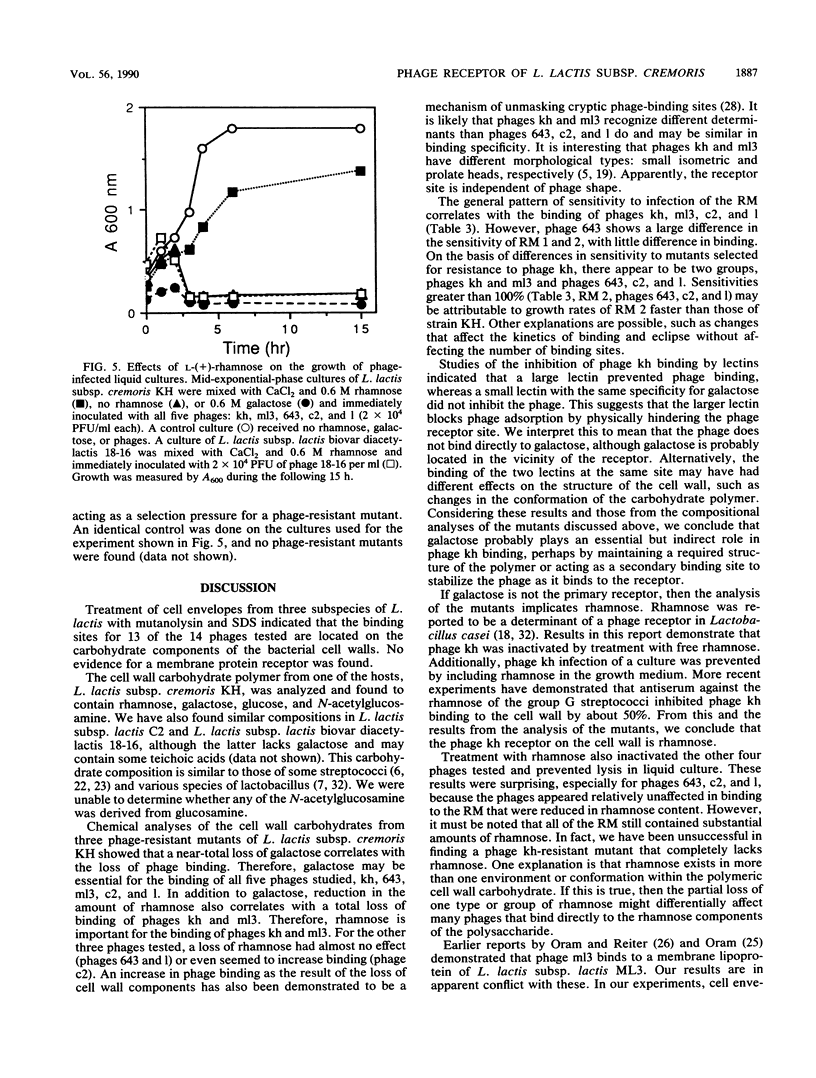
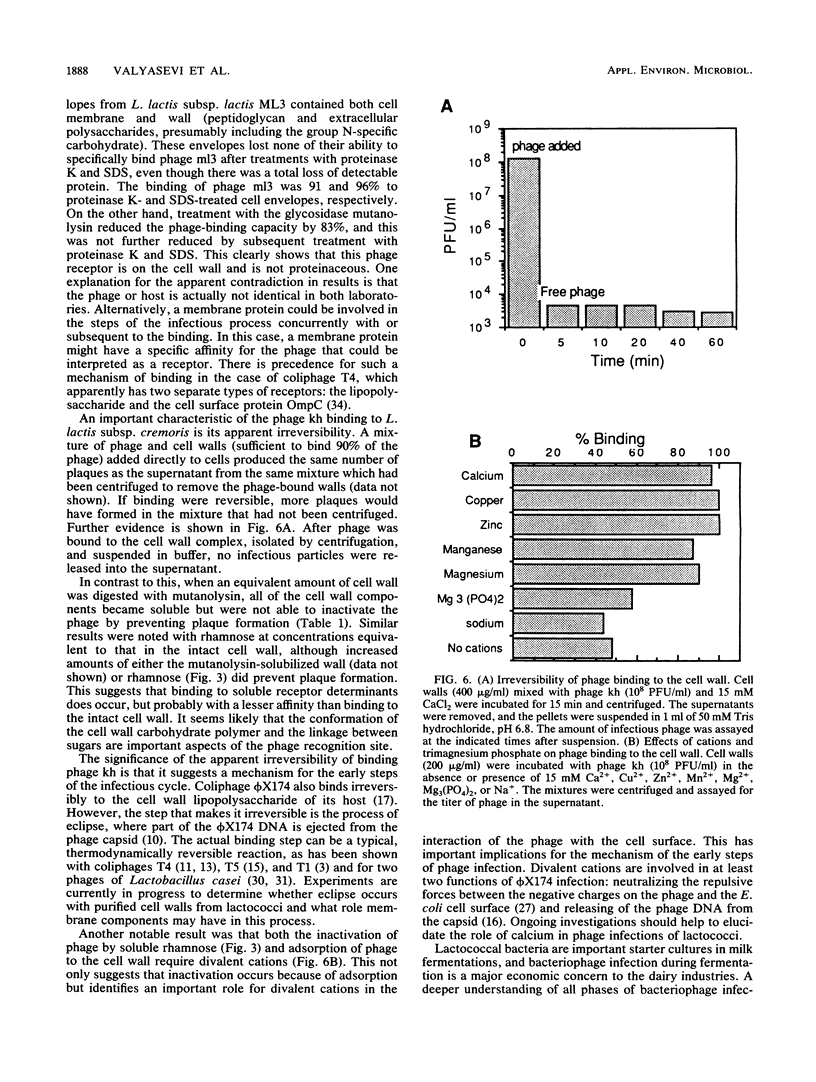
A receptor for bacteriophages of lactic acid bacteria, including Lactococcus lactis subsp. cremoris KH, was found on the cell wall and not on the cell membrane, as determined by a phage-binding assay of sodium dodecyl sulfate- and mutanolysin-treated cell walls. The cell wall carbohydrates of L. lactis subsp. cremoris KH were analyzed by gas chromatography and mass spectrometry and found to contain rhamnose, galactose, glucose and N-acetylglucosamine. Similar analysis of mutants that were reduced in the ability to bind phages kh, 643, c2, ml3, and 1 indicated that galactose was essential for binding all phages. In addition, rhamnose was required for binding phages kh and ml3. Inhibition studies of phage binding by using two different lectins with a specificity for galactose indicated that phage kh may not bind directly to galactose. Rather, galactose may be an essential structural component located in the vicinity of the receptor. Incubation of any of the five phages with rhamnose or of phage kh with purified cell walls inactivated the phages. Inactivation required divalent cations and was irreversible. Inactivation of phages was stereospecific for rhamnose, as neither L-(+)- nor D-(-)-fucose (the stereoisomers of rhamnose) inhibited the phage. Furthermore, phage infection of a culture was completely inhibited by the addition of rhamnose to the medium. Therefore, the receptor for phage kh appears to be a rhamnose component of the extracellular wall polysaccharide.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CURTIS S. N., KRAUSE R. M. ANTIGENIC RELATIONSHIPS BETWEEN GROUPS B AND G STREPTOCOCCI. J Exp Med. 1964 Oct 1;120:629–637. doi: 10.1084/jem.120.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee A. N. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J Bacteriol. 1969 May;98(2):519–527. doi: 10.1128/jb.98.2.519-527.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J. R. The kinetics of reversible and irreversible attachment of bacteriophage T-1. Virology. 1965 Aug;26(4):727–737. doi: 10.1016/0042-6822(65)90336-3. [DOI] [PubMed] [Google Scholar]
- Cleary P. P., Wannamaker L. W., Fisher M., Laible N. Studies of the receptor for phage A25 in group A streptococci: the role of peptidoglycan in reversible adsorption. J Exp Med. 1977 Mar 1;145(3):578–593. doi: 10.1084/jem.145.3.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas L. J., Wolin M. J. Cell wall polymers and phage lysis of Lactobacillus plantarum. Biochemistry. 1971 Apr 27;10(9):1551–1555. doi: 10.1021/bi00785a007. [DOI] [PubMed] [Google Scholar]
- FUJIMURA R., KAESBERG P. The adsorption of bacteriophage phi-X174 to its host. Biophys J. 1962 Nov;2:433–449. doi: 10.1016/s0006-3495(62)86866-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischetti V. A., Zabriskie J. B. Studies on streptococcal bacteriophages. II. Adsorption studies on group A and group C streptococcal bacteriophages. J Exp Med. 1968 Mar 1;127(3):489–505. doi: 10.1084/jem.127.3.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A., Morgan S. L., Hudson J. R., Zhu Z. T., Lau P. Y. Capillary gas chromatographic analysis of alditol acetates of neutral and amino sugars in bacterial cell walls. J Chromatogr. 1983 Feb 18;256(3):429–438. doi: 10.1016/s0021-9673(01)88260-1. [DOI] [PubMed] [Google Scholar]
- Furukawa H., Mizushima S. Roles of cell surface components of Escherichia coli K-12 in bacteriophage T4 infection: interaction of tail core with phospholipids. J Bacteriol. 1982 May;150(2):916–924. doi: 10.1128/jb.150.2.916-924.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehring K., Charbit A., Brissaud E., Hofnung M. Bacteriophage lambda receptor site on the Escherichia coli K-12 LamB protein. J Bacteriol. 1987 May;169(5):2103–2106. doi: 10.1128/jb.169.5.2103-2106.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller K., Braun V. Accelerated adsorption of bacteriophage T5 to Escherichia coli F, resulting from reversible tail fiber-lipopolysaccharide binding. J Bacteriol. 1979 Jul;139(1):32–38. doi: 10.1128/jb.139.1.32-38.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona N. L. Mechanism of adsorption and eclipse of bacteriophage phi chi 174. 3. Comparison of the activation parameters for the in vitro and in vivo eclipse reactions with mutant and wild-type virus. J Virol. 1974 Sep;14(3):469–478. doi: 10.1128/jvi.14.3.469-478.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona N. L., Selvidge L. Mechanism of adsorption and eclipse of bacteriophage phi X174. II. Attachment and eclipse with isolated Escherichia coli cell wall lipopolysaccharide. J Virol. 1973 May;11(5):775–782. doi: 10.1128/jvi.11.5.775-782.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis A. W., Klaenhammer T. R. Bacteriophage Resistance Conferred on Lactic Streptococci by the Conjugative Plasmid pTR2030: Effects on Small Isometric-, Large Isometric-, and Prolate-Headed Phages. Appl Environ Microbiol. 1986 Jun;51(6):1272–1277. doi: 10.1128/aem.51.6.1272-1277.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazwinski S. M., Lindberg A. A., Kornberg A. The lipopolysaccharide receptor for bacteriophage phiX174 and S13. Virology. 1975 Jul;66(1):268–282. doi: 10.1016/0042-6822(75)90197-x. [DOI] [PubMed] [Google Scholar]
- KRAUSE R. M. SYMPOSIUM ON RELATIONSHIP OF STRUCTURE OF MICROORGANISMS TO THEIR IMMUNOLOGICAL PROPERTIES. IV. ANTIGENIC AND BIOCHEMICAL COMPOSITION OF HEMOLYTIC STREPTOCOCCAL CELL WALLS. Bacteriol Rev. 1963 Dec;27:369–380. doi: 10.1128/br.27.4.369-380.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh B. P., Pettingill G. Adsorption of Bacteriophage eb7 on Streptococcus cremoris EB7. Appl Environ Microbiol. 1983 Jun;45(6):1946–1948. doi: 10.1128/aem.45.6.1946-1948.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz E., Ghuysen J. M., Heymann H. Cell walls of Streptococcus pyogenes, type 14. C polysaccharide-peptidoglycan and G polysaccharide-peptidoglycan complexes. Biochemistry. 1967 Dec;6(12):3659–3670. doi: 10.1021/bi00864a007. [DOI] [PubMed] [Google Scholar]
- Newbold J. E., Sinsheimer R. L. Process of infection with bacteriophage phi-X174. XXXIV. Kinetic of the attachment and eclipse steps of the infection. J Virol. 1970 Apr;5(4):427–431. doi: 10.1128/jvi.5.4.427-431.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oram J. D. Isolation and properties of a phage receptor substance from the plasma membrane of Streptococcus lactis ML 3. J Gen Virol. 1971 Oct;13(1):59–71. doi: 10.1099/0022-1317-13-1-59. [DOI] [PubMed] [Google Scholar]
- Oram J. D., Reiter B. The adsorption of phage to group N streptococci. The specificity of adsorption and the location of phage receptor substances in cell-wall and plasma-membrane fractions. J Gen Virol. 1968 Jul;3(1):103–119. doi: 10.1099/0022-1317-3-1-103. [DOI] [PubMed] [Google Scholar]
- Rowatt E. The role of bivalent ions in the inactivation of bacteriophage phi X174 by lipopolysaccharide from Escherichia coli C. Biochem J. 1984 Oct 1;223(1):23–29. doi: 10.1042/bj2230023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sijtsma L., Sterkenburg A., Wouters J. T. Properties of the Cell Walls of Lactococcus lactis subsp. cremoris SK110 and SK112 and Their Relation to Bacteriophage Resistance. Appl Environ Microbiol. 1988 Nov;54(11):2808–2811. doi: 10.1128/aem.54.11.2808-2811.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terzaghi B. E., Sandine W. E. Improved medium for lactic streptococci and their bacteriophages. Appl Microbiol. 1975 Jun;29(6):807–813. doi: 10.1128/am.29.6.807-813.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe K., Takesue S. Use of L-rhamnose to study irreversible adsorption of bacteriophage PL-1 to a strain of Lactobacillus casei. J Gen Virol. 1975 Jul;28(1):29–35. doi: 10.1099/0022-1317-28-1-29. [DOI] [PubMed] [Google Scholar]
- Yokokura T. Phage receptor material in Lactobacillus casei cell wall. I. Effect of L-rhamnose on phage adsorption to the cell wall. Jpn J Microbiol. 1971 Sep;15(5):457–463. doi: 10.1111/j.1348-0421.1971.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Yokokura T. Phage receptor material in Lactobacillus casei. J Gen Microbiol. 1977 May;100(1):139–145. doi: 10.1099/00221287-100-1-139. [DOI] [PubMed] [Google Scholar]
- Young F. E. Requirement of glucosylated teichoic acid for adsorption of phage in Bacillus subtilis 168. Proc Natl Acad Sci U S A. 1967 Dec;58(6):2377–2384. doi: 10.1073/pnas.58.6.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu F., Mizushima S. Roles of lipopolysaccharide and outer membrane protein OmpC of Escherichia coli K-12 in the receptor function for bacteriophage T4. J Bacteriol. 1982 Aug;151(2):718–722. doi: 10.1128/jb.151.2.718-722.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]


