Abstract
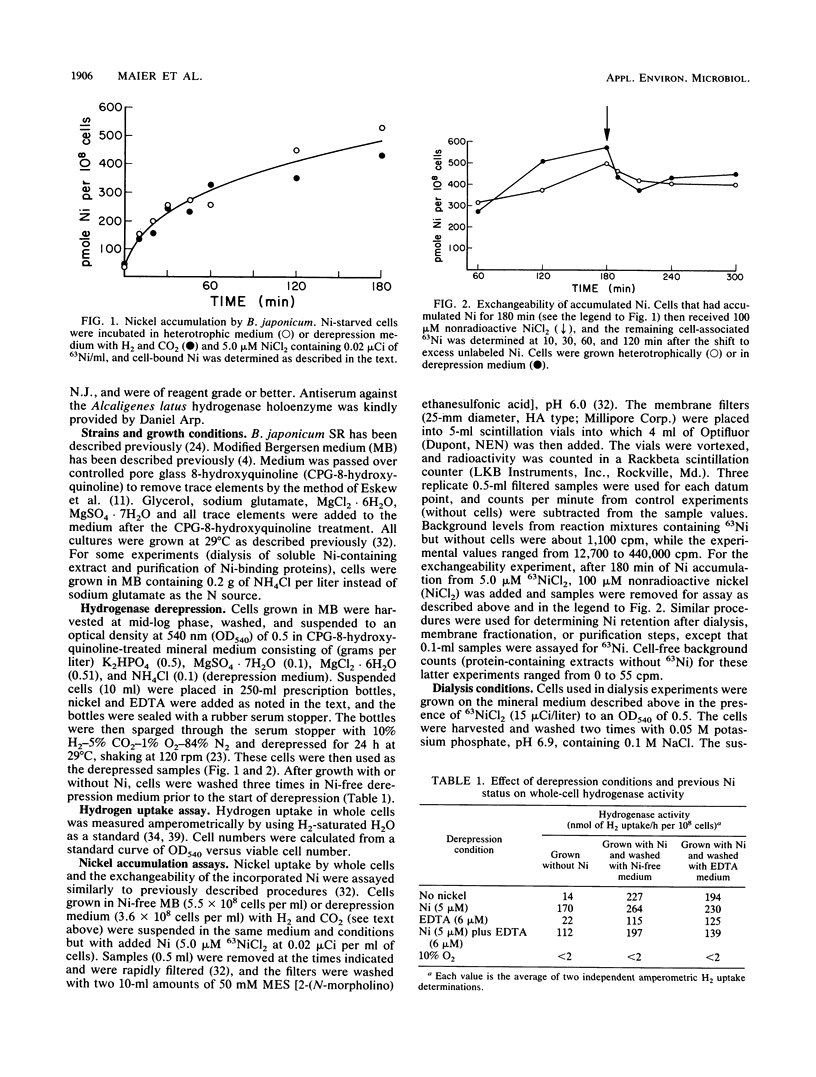
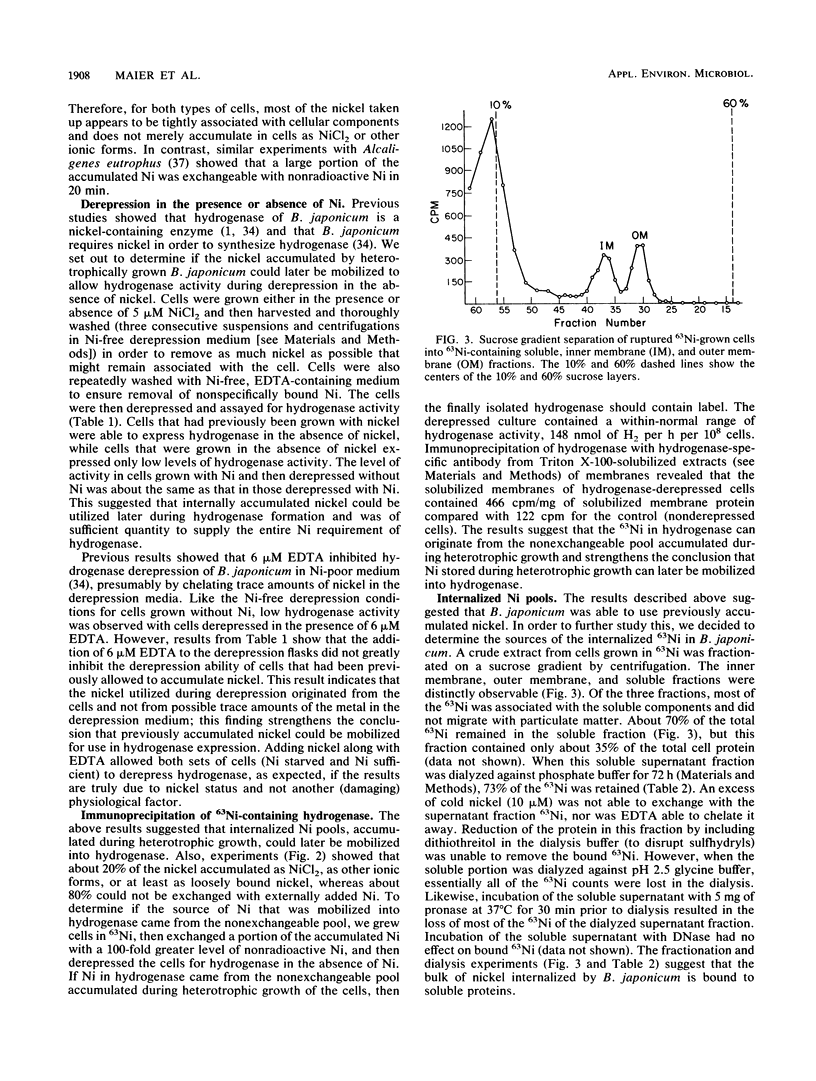
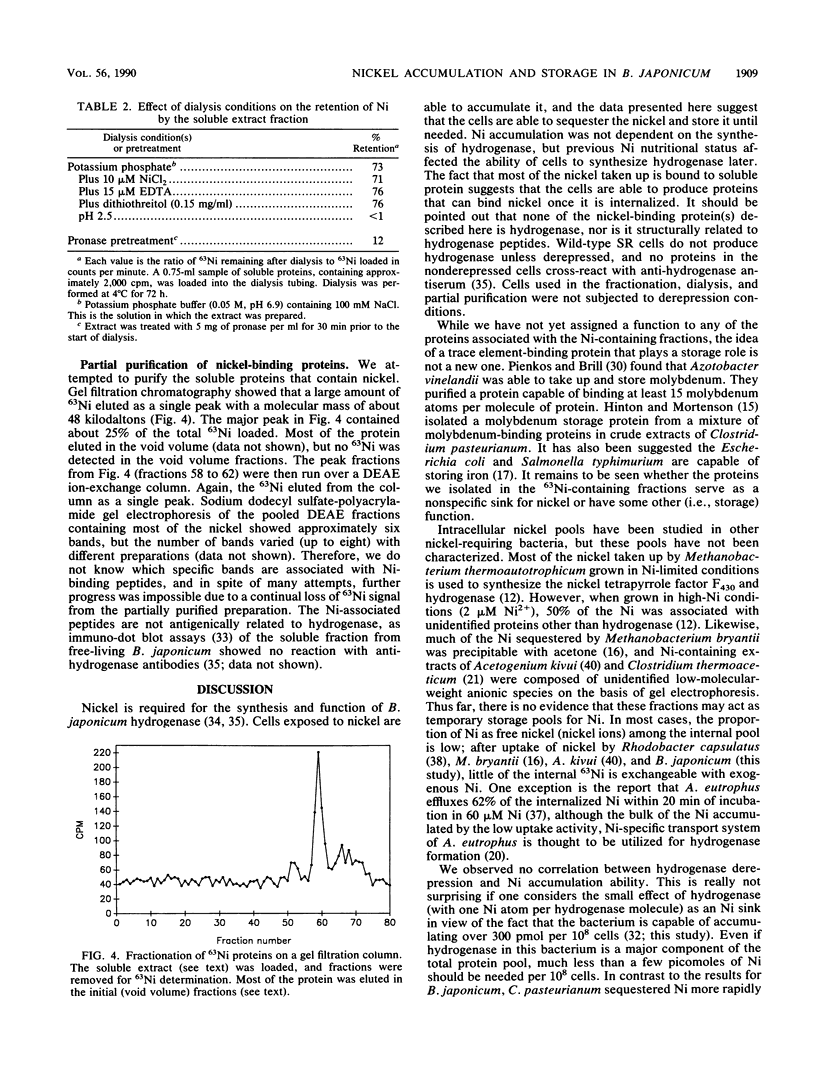
Hydrogenase-derepressed (chemolithotrophic growth conditions) and heterotrophically grown cultures of Bradyrhizobium japonicum accumulated nickel about equally over a 3-h period. Both types of cultures accumulated nickel primarily in a form that was not exchangeable with NiCl2, and they accumulated much more Ni than would be needed for the Ni-containing hydrogenase. The nickel accumulated by heterotrophically incubated cultures could later be mobilized to allow active hydrogenase synthesis during derepression in the absence of nickel, while cells both grown and derepressed without nickel had low hydrogenase activities. The level of activity in cells grown with Ni and then derepressed without nickel was about the same as that in cultures derepressed in the presence of nickel. The Ni accumulated by heterotrophically grown cultures was associated principally with soluble proteins rather than particulate material, and this Ni was not lost upon dialyzing an extract containing the soluble proteins against either Ni-containing or EDTA-containing buffer. However, this Ni was lost upon pronase or low pH treatments. The soluble Ni-binding proteins were partially purified by gel filtration and DEAE chromatography. They were not antigenically related to hydrogenase peptides. Much of the 63Ni eluted as a single peak of 48 kilodaltons. Experiments involving immunoprecipitation of 63Ni-containing hydrogenase suggested that the stored source of Ni in heterotrophic cultures that could later be mobilized into hydrogenase resided in the nonexchangeable Ni-containing fraction rather than in loosely bound or ionic forms.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arp D. J., McCollum L. C., Seefeldt L. C. Molecular and immunological comparison of membrane-bound, H2-oxidizing hydrogenases of Bradyrhizobium japonicum, Alcaligenes eutrophus, Alcaligenes latus, and Azotobacter vinelandii. J Bacteriol. 1985 Jul;163(1):15–20. doi: 10.1128/jb.163.1.15-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arp D. J. Rhizobium japonicum hydrogenase: purification to homogeneity from soybean nodules, and molecular characterization. Arch Biochem Biophys. 1985 Mar;237(2):504–512. doi: 10.1016/0003-9861(85)90303-0. [DOI] [PubMed] [Google Scholar]
- BARTHA R., ORDAL E. J. NICKEL-DEPENDENT CHEMOLITHOTROPHIC GROWTH OF TWO HYDROGENOMONAS STRAINS. J Bacteriol. 1965 Apr;89:1015–1019. doi: 10.1128/jb.89.4.1015-1019.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop P. E., Guevara J. G., Engelke J. A., Evans H. J. Relation between Glutamine Synthetase and Nitrogenase Activities in the Symbiotic Association between Rhizobium japonicum and Glycine max. Plant Physiol. 1976 Apr;57(4):542–546. doi: 10.1104/pp.57.4.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bryson M. F., Drake H. L. Energy-dependent transport of nickel by Clostridium pasteurianum. J Bacteriol. 1988 Jan;170(1):234–238. doi: 10.1128/jb.170.1.234-238.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell P. M., Smith G. D. Transport and accumulation of nickel ions in the cyanobacterium Anabaena cylindrica. Arch Biochem Biophys. 1986 Feb 1;244(2):470–477. doi: 10.1016/0003-9861(86)90615-6. [DOI] [PubMed] [Google Scholar]
- Eberz G., Eitinger T., Friedrich B. Genetic determinants of a nickel-specific transport system are part of the plasmid-encoded hydrogenase gene cluster in Alcaligenes eutrophus. J Bacteriol. 1989 Mar;171(3):1340–1345. doi: 10.1128/jb.171.3.1340-1345.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskew D. L., Welch R. M., Cary E. E. A simple plant nutrient solution purification method for effective removal of trace metals using controlled pore glass-8-hydroxyquinoline chelation column chromatography. Plant Physiol. 1984 Sep;76(1):103–105. doi: 10.1104/pp.76.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton S. M., Mortenson L. E. Identification of molybdoproteins in Clostridium pasteurianum. J Bacteriol. 1985 May;162(2):477–484. doi: 10.1128/jb.162.2.477-484.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell K. F., Sprott G. D. Nickel transport in Methanobacterium bryantii. J Bacteriol. 1982 Sep;151(3):1195–1203. doi: 10.1128/jb.151.3.1195-1203.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lundie L. L., Jr, Yang H. C., Heinonen J. K., Dean S. I., Drake H. L. Energy-dependent, high-affinity transport of nickel by the acetogen Clostridium thermoaceticum. J Bacteriol. 1988 Dec;170(12):5705–5708. doi: 10.1128/jb.170.12.5705-5708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Hanus F. J., Evans H. J. Regulation of hydrogenase in Rhizobium japonicum. J Bacteriol. 1979 Feb;137(2):825–829. doi: 10.1128/jb.137.2.825-829.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Merberg D. M. Rhizobium japonicum mutants that are hypersensitive to repression of H2 uptake by oxygen. J Bacteriol. 1982 Apr;150(1):161–167. doi: 10.1128/jb.150.1.161-167.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merberg D., O'Hara E. B., Maier R. J. Regulation of hydrogenase in Rhizobium japonicum: analysis of mutants altered in regulation by carbon substrates and oxygen. J Bacteriol. 1983 Dec;156(3):1236–1242. doi: 10.1128/jb.156.3.1236-1242.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P. D., Maier R. J. Hydrogenase synthesis in Bradyrhizobium japonicum Hupc mutants is altered in sensitivity to DNA gyrase inhibitors. Appl Environ Microbiol. 1989 May;55(5):1157–1164. doi: 10.1128/aem.55.5.1157-1164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brian M. R., Maier R. J. Hydrogen metabolism in Rhizobium: energetics, regulation, enzymology and genetics. Adv Microb Physiol. 1988;29:1–52. doi: 10.1016/s0065-2911(08)60345-8. [DOI] [PubMed] [Google Scholar]
- Pienkos P. T., Brill W. J. Molybdenum accumulation and storage in Klebsiella pneumoniae and Azotobacter vinelandii. J Bacteriol. 1981 Feb;145(2):743–751. doi: 10.1128/jb.145.2.743-751.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polacheck I., Cabib E. A simple procedure for protein determination by the Lowry method in dilute solutions and in the presence of interfering substances. Anal Biochem. 1981 Nov 1;117(2):311–314. doi: 10.1016/0003-2697(81)90784-3. [DOI] [PubMed] [Google Scholar]
- Siddiqui R. A., Schlegel H. G., Meyer M. Inducible and constitutive expression of pMOL28-encoded nickel resistance in Alcaligenes eutrophus N9A. J Bacteriol. 1988 Sep;170(9):4188–4193. doi: 10.1128/jb.170.9.4188-4193.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults L. W., Mallick S., Maier R. J. Nickel uptake in Bradyrhizobium japonicum. J Bacteriol. 1987 Apr;169(4):1398–1402. doi: 10.1128/jb.169.4.1398-1402.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults L. W., Moshiri F., Maier R. J. Aerobic purification of hydrogenase from Rhizobium japonicum by affinity chromatography. J Bacteriol. 1986 Jun;166(3):795–800. doi: 10.1128/jb.166.3.795-800.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stults L. W., O'Hara E. B., Maier R. J. Nickel is a component of hydrogenase in Rhizobium japonicum. J Bacteriol. 1984 Jul;159(1):153–158. doi: 10.1128/jb.159.1.153-158.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa M., Reid G. A. Preparation and use of antibodies against insoluble membrane proteins. Methods Enzymol. 1983;97:305–311. doi: 10.1016/0076-6879(83)97142-2. [DOI] [PubMed] [Google Scholar]
- Tabillion R., Kaltwasser H. Energieabhängige 63Ni-Aufnahme bei Alcaligenes eutrophus Stamm H1 und H16. Arch Microbiol. 1977 May 13;113(1-2):145–151. doi: 10.1007/BF00428595. [DOI] [PubMed] [Google Scholar]
- Yang H. C., Daniel S. L., Hsu T. D., Drake H. L. Nickel transport by the thermophilic acetogen Acetogenium kivui. Appl Environ Microbiol. 1989 May;55(5):1078–1081. doi: 10.1128/aem.55.5.1078-1081.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]


