Abstract
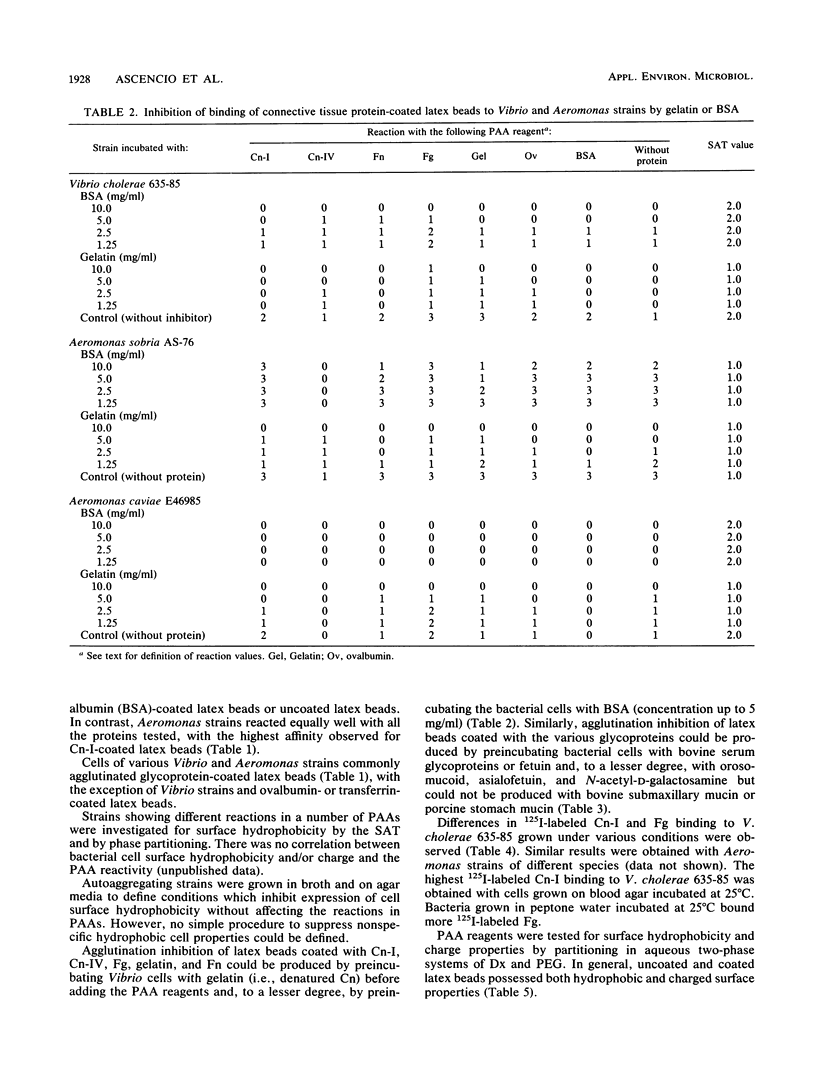
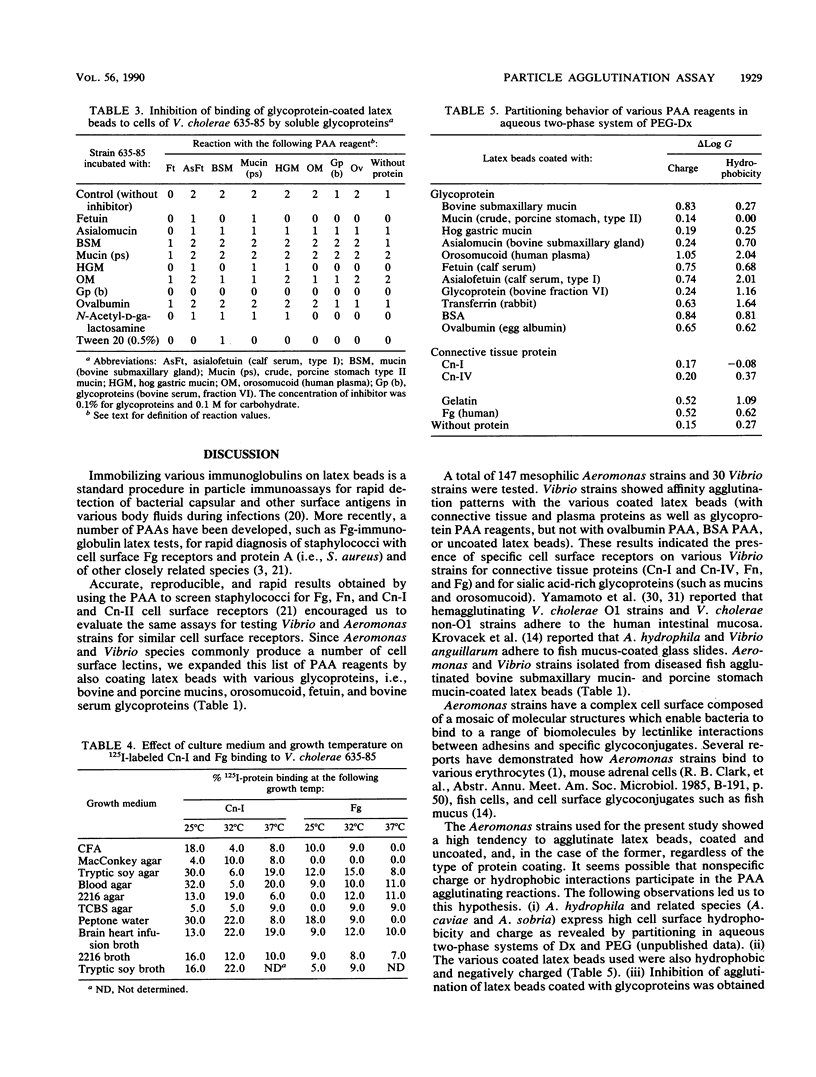
A rapid particle agglutination assay (PAA) utilizing latex beads coated with connective tissue and serum proteins was evaluated for its ability to identify fibronectin, collagen (types I and IV), fibrinogen, and transferrin cell surface receptors on Vibrio and Aeromonas strains isolated from diseased fish, human infections, and the environment. Similar tests were performed to screen for cell surface lectins. Vibrio as well as Aeromonas strains were found to bind connective tissue proteins (collagen types I, II, and IV and fibronectin), serum proteins (i.e., fibrinogen), and glycoproteins (bovine submaxillary mucin, hog gastric mucin, orosomucoid, and fetuin) immobilized on the latex particles. The specificity of the agglutination reaction was studied by particle agglutination inhibition assays performed by preincubating bacterial suspensions in solutions containing either gelatin (for the various connective tissue protein PAA reagents) or sialic acid-rich glycoproteins (for the various glycoprotein PAA reagents). Expression of cell surface receptors for connective tissue proteins was found to depend on culture methods.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson H. M., Trust T. J. Hemagglutination properties and adherence ability of Aeromonas hydrophila. Infect Immun. 1980 Mar;27(3):938–946. doi: 10.1128/iai.27.3.938-946.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essers L., Radebold K. Rapid and reliable identification of Staphylococcus aureus by a latex agglutination test. J Clin Microbiol. 1980 Nov;12(5):641–643. doi: 10.1128/jcm.12.5.641-643.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröman G., Switalski L. M., Faris A., Wadström T., Hök M. Binding of Escherichia coli to fibronectin. A mechanism of tissue adherence. J Biol Chem. 1984 Dec 10;259(23):14899–14905. [PubMed] [Google Scholar]
- González E. A., Baloda S. B., Blanco J., Wadström T. Growth conditions for the expression of fibronectin and collagen binding to Salmonella. Zentralbl Bakteriol Mikrobiol Hyg A. 1988 Nov;269(4):437–446. doi: 10.1016/s0176-6724(88)80065-8. [DOI] [PubMed] [Google Scholar]
- Isaacs R. D., Paviour S. D., Bunker D. E., Lang S. D. Wound infection with aerogenic Aeromonas strains: a review of twenty-seven cases. Eur J Clin Microbiol Infect Dis. 1988 Jun;7(3):355–360. doi: 10.1007/BF01962336. [DOI] [PubMed] [Google Scholar]
- Johansson G. Partition of proteins and micro-organisms in aqueous biphasic systems. Mol Cell Biochem. 1974 Oct 30;4(3):169–180. doi: 10.1007/BF01731478. [DOI] [PubMed] [Google Scholar]
- Jonson G., Sanchez J., Svennerholm A. M. Expression and detection of different biotype-associated cell-bound haemagglutinins of Vibrio cholerae O1. J Gen Microbiol. 1989 Jan;135(1):111–120. doi: 10.1099/00221287-135-1-111. [DOI] [PubMed] [Google Scholar]
- Kabir S., Ali S. Characterization of surface properties of Vibrio cholerae. Infect Immun. 1983 Mar;39(3):1048–1058. doi: 10.1128/iai.39.3.1048-1058.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Källenius G., Möllby R., Svenson S. B., Helin I., Hultberg H., Cedergren B., Winberg J. Occurrence of P-fimbriated Escherichia coli in urinary tract infections. Lancet. 1981 Dec 19;2(8260-61):1369–1372. doi: 10.1016/s0140-6736(81)92797-5. [DOI] [PubMed] [Google Scholar]
- Larsen J. L., Mellergaard S. Agglutination Typing of Vibrio anguillarum Isolates from Diseased Fish and from the Environment. Appl Environ Microbiol. 1984 Jun;47(6):1261–1265. doi: 10.1128/aem.47.6.1261-1265.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungh A., Osterlind M., Wadström T. Cell surface hydrophobicity of group D and viridans streptococci isolated from patients with septicaemia. Zentralbl Bakteriol Mikrobiol Hyg A. 1986 May;261(3):280–286. doi: 10.1016/s0176-6724(86)80045-1. [DOI] [PubMed] [Google Scholar]
- Lämmler C., Frede C., Gürtürk K., Hildebrand A., Blobel H. Binding activity of Streptococcus canis for albumin and other plasma proteins. J Gen Microbiol. 1988 Aug;134(8):2317–2323. doi: 10.1099/00221287-134-8-2317. [DOI] [PubMed] [Google Scholar]
- Markwell M. A. A new solid-state reagent to iodinate proteins. I. Conditions for the efficient labeling of antiserum. Anal Biochem. 1982 Sep 15;125(2):427–432. doi: 10.1016/0003-2697(82)90025-2. [DOI] [PubMed] [Google Scholar]
- Miller C. A., Patterson W. L., Johnson P. K., Swartzell C. T., Wogoman F., Albarella J. P., Carrico R. J. Detection of bacteria by hybridization of rRNA with DNA-latex and immunodetection of hybrids. J Clin Microbiol. 1988 Jul;26(7):1271–1276. doi: 10.1128/jcm.26.7.1271-1276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naidu A. S., Paulsson M., Wadström T. Particle agglutination assays for rapid detection of fibronectin, fibrinogen, and collagen receptors on Staphylococcus aureus. J Clin Microbiol. 1988 Aug;26(8):1549–1554. doi: 10.1128/jcm.26.8.1549-1554.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paula S. J., Duffey P. S., Abbott S. L., Kokka R. P., Oshiro L. S., Janda J. M., Shimada T., Sakazaki R. Surface properties of autoagglutinating mesophilic aeromonads. Infect Immun. 1988 Oct;56(10):2658–2665. doi: 10.1128/iai.56.10.2658-2665.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Research on Aeromonas and Plesiomonas. IV. Virulence factors. Experientia. 1987 Apr 15;43(4):367–374. [PubMed] [Google Scholar]
- Santos Y., Toranzo A. E., Barja J. L., Nieto T. P., Villa T. G. Virulence properties and enterotoxin production of Aeromonas strains isolated from fish. Infect Immun. 1988 Dec;56(12):3285–3293. doi: 10.1128/iai.56.12.3285-3293.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Statner B., Jones M. J., George W. L. Effect of incubation temperature on growth and soluble protein profiles of motile Aeromonas strains. J Clin Microbiol. 1988 Feb;26(2):392–393. doi: 10.1128/jcm.26.2.392-393.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart G. A., Bundell C. S., Burke V. Partial characterisation of a soluble haemagglutinin from human diarrhoeal isolates of Aeromonas. J Med Microbiol. 1986 Jun;21(4):319–324. doi: 10.1099/00222615-21-4-319. [DOI] [PubMed] [Google Scholar]
- Stoebner J. A., Payne S. M. Iron-regulated hemolysin production and utilization of heme and hemoglobin by Vibrio cholerae. Infect Immun. 1988 Nov;56(11):2891–2895. doi: 10.1128/iai.56.11.2891-2895.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trust T. J., Courtice I. D., Khouri A. G., Crosa J. H., Schiewe M. H. Serum resistance and hemagglutination ability of marine vibrios pathogenic for fish. Infect Immun. 1981 Dec;34(3):702–707. doi: 10.1128/iai.34.3.702-707.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Kamano T., Uchimura M., Iwanaga M., Yokota T. Vibrio cholerae O1 adherence to villi and lymphoid follicle epithelium: in vitro model using formalin-treated human small intestine and correlation between adherence and cell-associated hemagglutinin levels. Infect Immun. 1988 Dec;56(12):3241–3250. doi: 10.1128/iai.56.12.3241-3250.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T., Yokota T. Vibrio cholerae non-O1: production of cell-associated hemagglutinins and in vitro adherence to mucus coat and epithelial surfaces of the villi and lymphoid follicles of human small intestines treated with formalin. J Clin Microbiol. 1988 Oct;26(10):2018–2024. doi: 10.1128/jcm.26.10.2018-2024.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Man P., Cedergren B., Enerbäck S., Larsson A. C., Leffler H., Lundell A. L., Nilsson B., Svanborg-Edén C. Receptor-specific agglutination tests for detection of bacteria that bind globoseries glycolipids. J Clin Microbiol. 1987 Feb;25(2):401–406. doi: 10.1128/jcm.25.2.401-406.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]


