Abstract
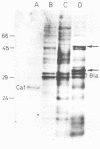
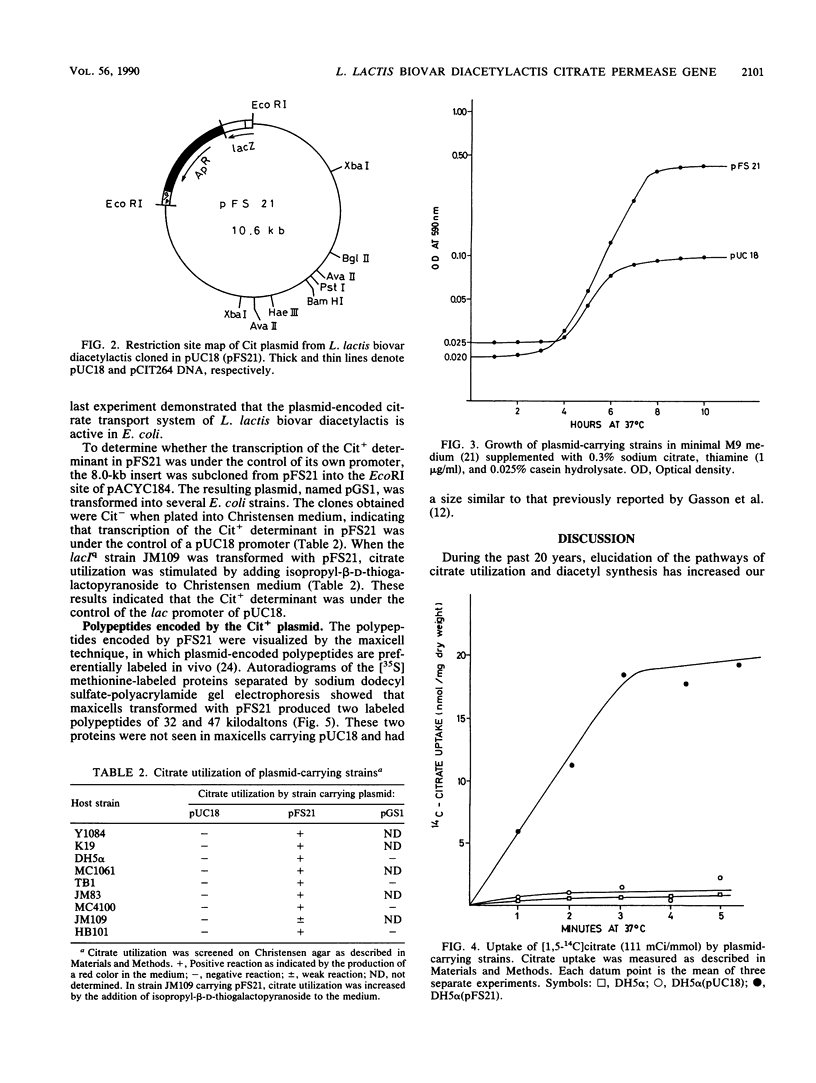
The citrate plasmid (Cit+ plasmid) from Lactococcus lactis subsp. lactis biovar diacetylactis was cloned into the EcoRI site of plasmid pUC18. This recombinant plasmid enabled Escherichia coli K-12 to transport and utilize citrate as a source of energy, indicating expression of the citrate permease from L. lactis biovar diacetylactis. The citrate permease was under the control of the lac promoter of pUC18. Genetic expression of the Cit+ plasmid in maxicells revealed that the plasmid encoded two polypeptides of 47 and 32 kilodaltons, determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. G., McKay L. L. Simple and rapid method for isolating large plasmid DNA from lactic streptococci. Appl Environ Microbiol. 1983 Sep;46(3):549–552. doi: 10.1128/aem.46.3.549-552.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baigorí M., Sesma F., de Ruiz Holgado A. P., de Mendoza D. Transfer of Plasmids between Bacillus subtilis and Streptococcus lactis. Appl Environ Microbiol. 1988 May;54(5):1309–1311. doi: 10.1128/aem.54.5.1309-1311.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns D. M., Beacham I. R. A method for the ligation of DNA following isolation from low melting temperature agarose. Anal Biochem. 1983 Nov;135(1):48–51. doi: 10.1016/0003-2697(83)90728-5. [DOI] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark D. Regulation of fatty acid degradation in Escherichia coli: analysis by operon fusion. J Bacteriol. 1981 Nov;148(2):521–526. doi: 10.1128/jb.148.2.521-526.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogan T. M. Citrate utilization in milk by Leuconostoc cremoris and Streptococcus diacetilactis. J Dairy Res. 1975 Feb;42(1):139–146. doi: 10.1017/s0022029900015168. [DOI] [PubMed] [Google Scholar]
- Gramajo H. C., Viale A. M., de Mendoza D. Expression of cloned genes by in vivo insertion of tac promoter using a mini-Mu bacteriophage. Gene. 1988 May 30;65(2):305–314. doi: 10.1016/0378-1119(88)90467-2. [DOI] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Characterization of Plasmid Deoxyribonucleic Acid in Streptococcus lactis subsp. diacetylactis: Evidence for Plasmid-Linked Citrate Utilization. Appl Environ Microbiol. 1979 Feb;37(2):316–323. doi: 10.1128/aem.37.2.316-323.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempler G. M., McKay L. L. Improved Medium for Detection of Citrate-Fermenting Streptococcus lactis subsp. diacetylactis. Appl Environ Microbiol. 1980 Apr;39(4):926–927. doi: 10.1128/aem.39.4.926-927.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LARA F. J. S., STOKES J. L. Oxidation of citrate by Escherichia coli. J Bacteriol. 1952 Mar;63(3):415–420. doi: 10.1128/jb.63.3.415-420.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Reynolds C. H., Silver S. Citrate utilization by Escherichia coli: plasmid- and chromosome-encoded systems. J Bacteriol. 1983 Dec;156(3):1019–1024. doi: 10.1128/jb.156.3.1019-1024.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancar A., Hack A. M., Rupp W. D. Simple method for identification of plasmid-coded proteins. J Bacteriol. 1979 Jan;137(1):692–693. doi: 10.1128/jb.137.1.692-693.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- de Mendoza D., Klages Ulrich A., Cronan J. E., Jr Thermal regulation of membrane fluidity in Escherichia coli. Effects of overproduction of beta-ketoacyl-acyl carrier protein synthase I. J Biol Chem. 1983 Feb 25;258(4):2098–2101. [PubMed] [Google Scholar]