Abstract
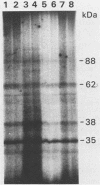
The possible involvement of protein phosphorylation in the clostridial stress response was investigated by radioactively labeling growing cells of Clostridium acetobutylicum with 32Pi or cell extracts with [gamma-32P]ATP. Several phosphoproteins were identified; these were not affected by the growth stage of the culture. Although the extent of protein phosphorylation was increased by heat stress, the phosphoproteins did not correspond to known stress proteins seen in one-dimensional sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Purified clostridial DnaK, a stress protein, acted as a kinase catalyzing the phosphorylation of a 50-kilodalton protein. The phosphorylation of this protein was enhanced in extracts prepared from heat-stressed cells. Diadenosine-5',5"'-P1,P4-tetraphosphate had no influence on protein phosphorylation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balodimos I. A., Kashket E. R., Rapaport E. Metabolism of adenylylated nucleotides in Clostridium acetobutylicum. J Bacteriol. 1988 May;170(5):2301–2305. doi: 10.1128/jb.170.5.2301-2305.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bochner B. R., Lee P. C., Wilson S. W., Cutler C. W., Ames B. N. AppppA and related adenylylated nucleotides are synthesized as a consequence of oxidation stress. Cell. 1984 May;37(1):225–232. doi: 10.1016/0092-8674(84)90318-0. [DOI] [PubMed] [Google Scholar]
- Bochner B. R., Zylicz M., Georgopoulos C. Escherichia coli DnaK protein possesses a 5'-nucleotidase activity that is inhibited by AppppA. J Bacteriol. 1986 Nov;168(2):931–935. doi: 10.1128/jb.168.2.931-935.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B., Walker G. C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bacteriol. 1989 May;171(5):2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cegielska A., Georgopoulos C. Biochemical properties of the Escherichia coli dnaK heat shock protein and its mutant derivatives. Biochimie. 1989 Sep-Oct;71(9-10):1071–1077. doi: 10.1016/0300-9084(89)90113-2. [DOI] [PubMed] [Google Scholar]
- Cortay J. C., Rieul C., Duclos B., Cozzone A. J. Characterization of the phosphoproteins of Escherichia coli cells by electrophoretic analysis. Eur J Biochem. 1986 Sep 1;159(2):227–237. doi: 10.1111/j.1432-1033.1986.tb09858.x. [DOI] [PubMed] [Google Scholar]
- Cozzone A. J. Protein phosphorylation in prokaryotes. Annu Rev Microbiol. 1988;42:97–125. doi: 10.1146/annurev.mi.42.100188.000525. [DOI] [PubMed] [Google Scholar]
- Farr S. B., Arnosti D. N., Chamberlin M. J., Ames B. N. An apaH mutation causes AppppA to accumulate and affects motility and catabolite repression in Escherichia coli. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5010–5014. doi: 10.1073/pnas.86.13.5010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutkins R. W., Kashket E. R. Phosphotransferase Activity in Clostridium acetobutylicum from Acidogenic and Solventogenic Phases of Growth. Appl Environ Microbiol. 1986 May;51(5):1121–1123. doi: 10.1128/aem.51.5.1121-1123.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr D. B., Emerich D. W. Protein phosphorylation in Bradyrhizobium japonicum bacteroids and cultures. J Bacteriol. 1989 Jun;171(6):3420–3426. doi: 10.1128/jb.171.6.3420-3426.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. A., Simon L. D. Divergent effects of a dnaK mutation on abnormal protein degradation in Escherichia coli. Mol Microbiol. 1988 Jan;2(1):31–41. doi: 10.1111/j.1365-2958.1988.tb00004.x. [DOI] [PubMed] [Google Scholar]
- Köhler E., Antranikian G. Covalent modification of proteins in Bacillus subtilis during the process of sporulation, germination and outgrowth. FEMS Microbiol Lett. 1989 Jan 1;48(1):87–92. doi: 10.1016/0378-1097(89)90152-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Londesborough J. Phosphorylation of proteins in Clostridium thermohydrosulfuricum. J Bacteriol. 1986 Feb;165(2):595–601. doi: 10.1128/jb.165.2.595-601.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plateau P., Fromant M., Blanquet S. Heat shock and hydrogen peroxide responses of Escherichia coli are not changed by dinucleoside tetraphosphate hydrolase overproduction. J Bacteriol. 1987 Aug;169(8):3817–3820. doi: 10.1128/jb.169.8.3817-3820.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reizer J., Saier M. H., Jr, Deutscher J., Grenier F., Thompson J., Hengstenberg W. The phosphoenolpyruvate:sugar phosphotransferase system in gram-positive bacteria: properties, mechanism, and regulation. Crit Rev Microbiol. 1988;15(4):297–338. doi: 10.3109/10408418809104461. [DOI] [PubMed] [Google Scholar]
- Rieul C., Cortay J. C., Bleicher F., Cozzone A. J. Effect of bacteriophage M13 infection on phosphorylation of dnaK protein and other Escherichia coli proteins. Eur J Biochem. 1987 Nov 2;168(3):621–627. doi: 10.1111/j.1432-1033.1987.tb13461.x. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y. The dnaK gene of Escherichia coli functions in initiation of chromosome replication. J Bacteriol. 1988 Feb;170(2):972–979. doi: 10.1128/jb.170.2.972-979.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stock J. B., Ninfa A. J., Stock A. M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989 Dec;53(4):450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowe D. J., Atkinson T., Mann N. H. Protein kinase activities in cell-free extracts of Streptomyces coelicolor A3(2). Biochimie. 1989 Sep-Oct;71(9-10):1101–1105. doi: 10.1016/0300-9084(89)90117-x. [DOI] [PubMed] [Google Scholar]
- Terracciano J. S., Kashket E. R. Intracellular Conditions Required for Initiation of Solvent Production by Clostridium acetobutylicum. Appl Environ Microbiol. 1986 Jul;52(1):86–91. doi: 10.1128/aem.52.1.86-91.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terracciano J. S., Rapaport E., Kashket E. R. Stress- and Growth Phase-Associated Proteins of Clostridium acetobutylicum. Appl Environ Microbiol. 1988 Aug;54(8):1989–1995. doi: 10.1128/aem.54.8.1989-1995.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilly K., McKittrick N., Zylicz M., Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1983 Sep;34(2):641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- VanBogelen R. A., Kelley P. M., Neidhardt F. C. Differential induction of heat shock, SOS, and oxidation stress regulons and accumulation of nucleotides in Escherichia coli. J Bacteriol. 1987 Jan;169(1):26–32. doi: 10.1128/jb.169.1.26-32.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada M., Sekine K., Itikawa H. Participation of the dnaK and dnaJ gene products in phosphorylation of glutaminyl-tRNA synthetase and threonyl-tRNA synthetase of Escherichia coli K-12. J Bacteriol. 1986 Oct;168(1):213–220. doi: 10.1128/jb.168.1.213-220.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zylicz M., Georgopoulos C. Purification and properties of the Escherichia coli dnaK replication protein. J Biol Chem. 1984 Jul 25;259(14):8820–8825. [PubMed] [Google Scholar]
- Zylicz M., LeBowitz J. H., McMacken R., Georgopoulos C. The dnaK protein of Escherichia coli possesses an ATPase and autophosphorylating activity and is essential in an in vitro DNA replication system. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6431–6435. doi: 10.1073/pnas.80.21.6431. [DOI] [PMC free article] [PubMed] [Google Scholar]