Abstract
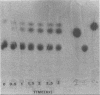
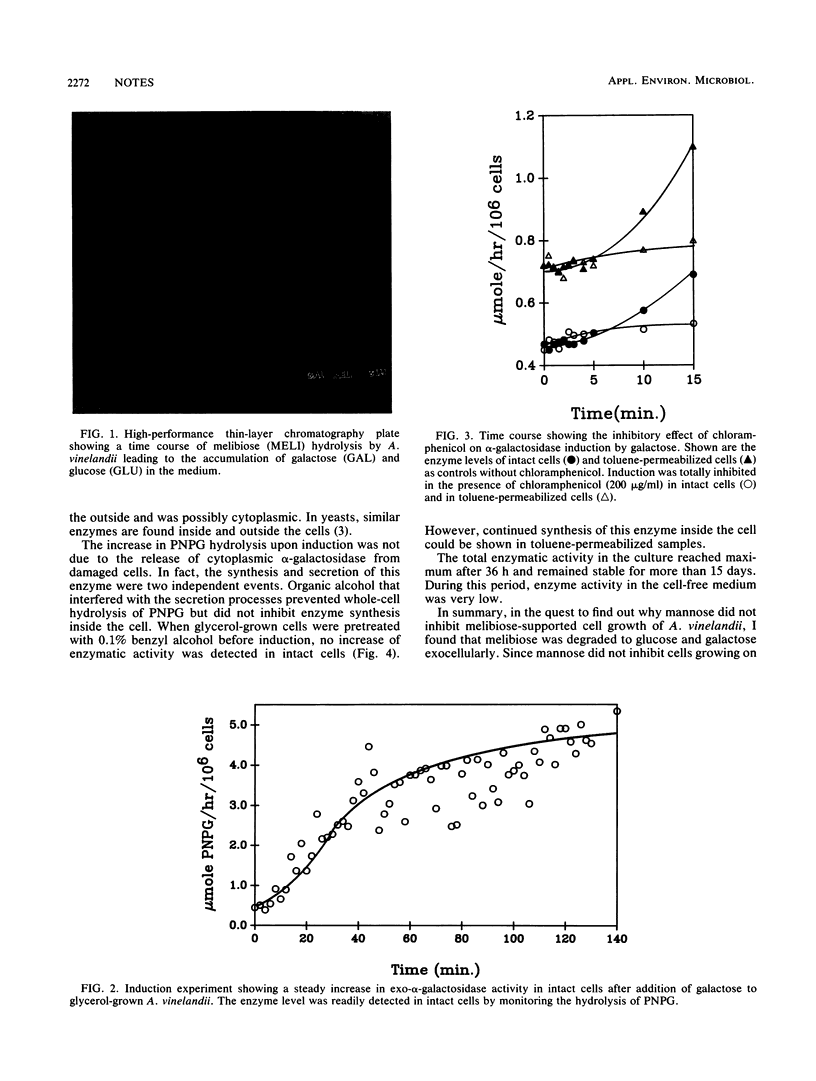
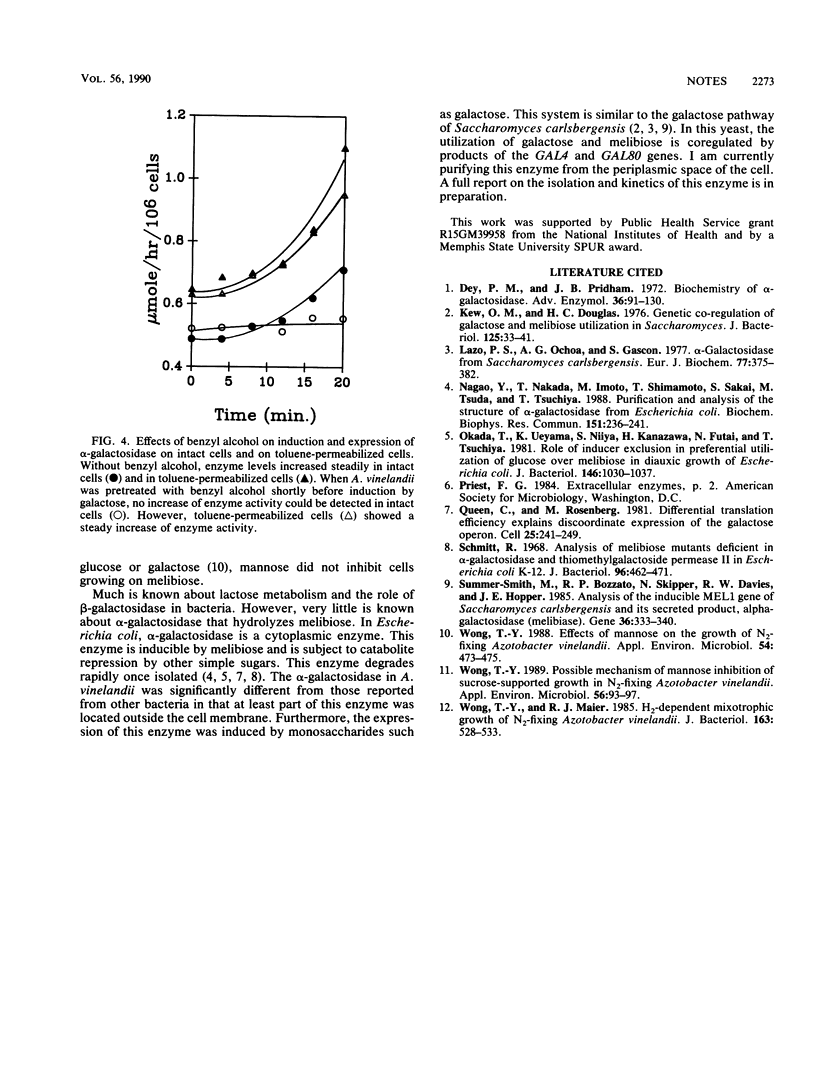
Azotobacter vinelandii hydrolyzed melibiose exocellularly, leading to an accumulation of free glucose and galactose in the medium. This enzyme could also be induced by galactose, raffinose, and stachyose. The alpha-galactosidase activity could be detected quantitatively by using p-nitrophenyl-alpha-galactopyranoside as a substrate for intact cells. Chloramphenicol totally inhibited the induction of this enzyme. However, benzyl alcohol inhibited the secretion of this enzyme but did not inhibit the biosynthesis of the enzyme.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dey P. M., Pridham J. B. Biochemistry of -galactosidases. Adv Enzymol Relat Areas Mol Biol. 1972;36:91–130. doi: 10.1002/9780470122815.ch3. [DOI] [PubMed] [Google Scholar]
- Kew O. M., Douglas H. C. Genetic co-regulation of galactose and melibiose utilization in Saccharomyces. J Bacteriol. 1976 Jan;125(1):33–41. doi: 10.1128/jb.125.1.33-41.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazo P. S., Ochoa A. G., Gascón S. alpha-Galactosidase from Saccharomyces carlsbergensis. Cellular localization, and purification of the external enzyme. Eur J Biochem. 1977 Jul 15;77(2):375–382. doi: 10.1111/j.1432-1033.1977.tb11677.x. [DOI] [PubMed] [Google Scholar]
- Nagao Y., Nakada T., Imoto M., Shimamoto T., Sakai S., Tsuda M., Tsuchiya T. Purification and analysis of the structure of alpha-galactosidase from Escherichia coli. Biochem Biophys Res Commun. 1988 Feb 29;151(1):236–241. doi: 10.1016/0006-291x(88)90584-0. [DOI] [PubMed] [Google Scholar]
- Okada T., Ueyama K., Niiya S., Kanazawa H., Futai M., Tsuchiya T. Role of inducer exclusion in preferential utilization of glucose over melibiose in diauxic growth of Escherichia coli. J Bacteriol. 1981 Jun;146(3):1030–1037. doi: 10.1128/jb.146.3.1030-1037.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queen C., Rosenberg M. Differential translation efficiency explains discoordinate expression of the galactose operon. Cell. 1981 Jul;25(1):241–249. doi: 10.1016/0092-8674(81)90249-x. [DOI] [PubMed] [Google Scholar]
- Schmitt R. Analysis of melibiose mutants deficient in alpha-galactosidase and thiomethylgalactoside permease II in Escherichia coli K-12. J Bacteriol. 1968 Aug;96(2):462–471. doi: 10.1128/jb.96.2.462-471.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumner-Smith M., Bozzato R. P., Skipper N., Davies R. W., Hopper J. E. Analysis of the inducible MEL1 gene of Saccharomyces carlsbergensis and its secreted product, alpha-galactosidase (melibiase). Gene. 1985;36(3):333–340. doi: 10.1016/0378-1119(85)90188-x. [DOI] [PubMed] [Google Scholar]
- Wong T. Y. Effects of Mannose on the Growth of N(2)-Fixing Azotobacter vinelandii. Appl Environ Microbiol. 1988 Feb;54(2):473–475. doi: 10.1128/aem.54.2.473-475.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. Y., Maier R. J. H2-dependent mixotrophic growth of N2-fixing Azotobacter vinelandii. J Bacteriol. 1985 Aug;163(2):528–533. doi: 10.1128/jb.163.2.528-533.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong T. Y. Possible mechanism of mannose inhibition of sucrose-supported growth in N2-fixing Azotobacter vinelandii. Appl Environ Microbiol. 1990 Jan;56(1):93–97. doi: 10.1128/aem.56.1.93-97.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]