Abstract
Exposure of mammalian skin to UV light results in induced gene transcription, playing a role in inflammation, immunosuppression, and tumor promotion. One important group of transcription factors induced by UV radiation is composed of members of the Rel/NF-κB family, which are known to play a major role in the transcriptional activation of many genes encoding inflammatory cytokines, adhesion molecules, and viral proteins. However, the upstream events in the transduction of the UVB signal to Rel protein activity are, as yet, unknown. Here, we provide biochemical evidence that exposure of keratinocytes to UVB causes rapid association of tumor necrosis factor (TNF) receptor 1 with its downstream partner TRAF-2. The functional relevance of this association is demonstrated by experiments showing that expression of a dominant negative TNF receptor 1 or TRAF-2 protein inhibits UVB-induced Rel-dependent transcription. Inclusion of a neutralizing antibody toward TNFα has no effect on UVB activation of a Rel-responsive reporter gene. Therefore, UVB-induced activation of Rel proteins via TNF receptor 1, independent of ligand activation, is a key component in the UV response in keratinocytes.
Ultraviolet radiation elicits many adverse effects in the skin including erythema, aging, cell death, and cancer (1). UV radiation also elicits synthesis of a set of proteins with the intention of protecting the skin; the collective term describing UV-induced adaptations is known as the UV response (2, 3). At sea level, the UV response results mostly from irradiation by UVB wavelengths and partly from UVA wavelengths; biologically potent UVC is absorbed by the ozone layer and is therefore not of physiological significance.
One of the most studied groups of transcription factors induced by UV radiation are members of the Rel/NF-κB family. This family of transcription factors resides in the cytoplasm bound to inhibitory proteins such as IκB-α. Upon stimulation, the inhibitory protein undergoes phosphorylation and degradation, leading to the unmasking of nuclear translocation signals (4, 5). Rel proteins play an important role in the regulation of cell growth, programmed cell death, immune responses, and viral regulation (6).
We have demonstrated previously that keratinocytes utilize a Ras-independent pathway for the induction of Rel proteins following UVB irradiation, whereas irradiation with UVA elicits no such response (7). In addition, we and others (7–9) have shown that UVR-induced activation of Rel proteins is not mediated by protein kinase C (PKC) but is dependent upon tyrosine kinases. However, the upstream events in the transduction of the UVB signal to Rel protein activity are, as yet, unknown. More recently, activation, autophosphorylation, and aggregation of membrane-bound receptors for epidermal growth factor (EGF), interleukin 1 (IL-1), tumor necrosis factor α (TNFα), and insulin have been demonstrated as key events in the induction of AP-1-mediated gene transcription following UV irradiation (8–10). This has led to the hypothesis that ligand-independent activation of cell membrane receptors underlies the UV response, which in turn may be viewed as the combined result of activation of a number of different signaling pathways.
As a consequence, a strong dependence on cellular context may be expected and is in fact supported by the Ras-independent activation of Rel/NF-κB in keratinocytes (7) and noninvolvement of the EGF receptor in UVB-induced AP-1 activation in the JB6 epidermal cell line (11).
Membrane receptors belonging to the TNF family of receptors including TNF receptors 1 and 2, CD40 and Fas/APO-1, are all capable of signaling Rel/NF-κB activation. This is achieved by association of the intracellular receptor domain to a complex of cytoplasmic signal transducers. In the case of TNF receptors and CD40, TRAF-2 is the key factor mediating induced Rel/NF-κB activity (12, 13), and in cell culture, overexpression of TRAF-2 has been shown to be sufficient for NF-κB activation (12). In normal skin, the TNF receptor 1 is expressed in all cellular layers, whereas the TNF receptor 2 is not expressed (14). Furthermore, TNFα induces Rel/NF-κB activation, and many of the genes induced by TNFα treatment are also induced when cells are exposed to UVB (for example collagenase, ICAM, and stromelysin) (15). Interestingly, the TNF receptor 1 also elicits apoptosis by engagement of a set of intracellular signal transducers different from TRAF-2 (13).
Similarly, a characteristic response to UV irradiation in skin is the formation of apoptotic so-called sunburn cells.
In this study, we have examined the role of TNF receptor 1 as a mediator of the activation of Rel/NF-κB proteins by UV radiation in keratinocytes. The results demonstrate that ligand-independent activation of the TNF receptor 1 leading to recruitment of the transducer TRAF-2 is a key component in the UV response.
MATERIALS AND METHODS
Cell Lines and Plasmids.
The murine keratinocyte cell line Balb/MK was cultivated in MCDB 153 medium supplemented with 50 μM CaCl2, 0.1 mM ethanolamine, 0.1 mM phosphoethanolamine, 10 ng/ml EGF, 0.5 μg/ml insulin, 0.5% fetal calf serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin. The construction of the VLRTE.CAT plasmid has been described elsewhere (16). The TNF receptor expression vectors TR 205 and TR 394 were generous gifts from M. Krönke. The TRAF-2 expression vector pcDNA3 TRAF2 was a generous gift from D. Wallach. The dominant negative TRAF-2 expression vector TRAF-2 (87–501) was constructed by PCR amplification of human TRAF-2 cDNA from amino acid 87 to amino acid 501 using primers containing a BamHI site and an ATG codon, and a XbaI site, respectively. The PCR product was inserted into the pcDNA3 vector (Invitrogen).
Preparation of Nuclear Extracts and Electrophoretic Mobility Shift Assay (EMSA) Analysis.
Nuclear cell extracts from Balb/MK keratinocytes were prepared as described elsewhere (17). EMSA analysis was performed as previously described (7) with 8 μg of nuclear extract and 100 pg of 32P-labeled oligonucleotide corresponding to the 3′ half of VLTRE termed VLY with a sequence as follows: agctTAAACTTGTACTTTCCCtcga. The resulting protein–DNA complexes were resolved on a pre-electrophoresed 5% polyacrylamide gel (30:0.5) with 1× TGE (50 mM Tris/2.7 mM EDTA/380 mM glycine) as running buffer. Antibodies recognizing p65, p50,p52, cRel, and Rel B were purchased from Santa Cruz Biotechnology.
Treatments of Cells.
Cell cultures were exposed to a noncytotoxic dose of UVB using a bank of 4 TL12 (Philips) bulbs with a UVB emission 16 W/m2. For the duration of UV radiation, the culture medium was replaced with PBS. UV irradiation was measured using a DRC-100X radiometer (Spectroline, NY).
Immunoprecipitation and Western Blotting.
Balb/MK cells were irradiated with UVB as indicated, and protein extracts were prepared after 5 min. 200 μg of total protein was incubated with 1:100 dilution of TNF receptor antibody (anti- hTNF-BP1, R & D Systems) for 8 h. The extract was then incubated for 2 h with 10 μl of Sepharose beads, and proteins were resolved by SDS/12% polyacrylamide gel electrophoresis. Resolved proteins were transferred to nitrocellulose, and bands were revealed using ECL (Amersham) according to the manufacturer’s instructions. The TRAF-2 protein was immunochemically detected using a TRAF-2 antibody (N19, Santa Cruz) at a dilution of 1:5000.
RESULTS
UVB and TNFα Induce Protein–DNA Complexes in Keratinocytes with Similar Kinetics and Composition.
Using EMSA analysis, with a Rel binding sequence termed VLTRE, we have shown previously that phorbol 12-tetradecanoate 13-acetate (TPA) and UVB induce different protein–DNA complexes in Balb/MK keratinocytes (7). To compare the characteristics of protein–DNA complexes induced following UVB and TNFα treatment, we again employed EMSA analysis, which demonstrates that treatment of Balb/MK keratinocytes with TNFα results in DNA–protein complexes with similar mobilities to those seen in UVB-treated cells (Fig. 1). As before, TPA treatment resulted in the formation of three complexes designated 1, 2, and 3, whereas UVB irradiation resulted in the formation of two complexes comigrating with complexes 2 and 3 observed in nuclear extracts from TPA-treated cells. Treatment of cells with TNFα also resulted in two DNA–protein complexes comigrating with the UVB-induced bands. DNA–protein complexes induced by TNFα were present from 10 min to at least 5 h post-treatment with no change in number or mobility of bands (data not shown). This is similar to the rapid induction of UVB-induced complexes that occur within 5 min following UV radiation. Furthermore, EMSA analysis in the presence of Rel-specific antibodies showed that TNFα treatment, like UVB, induces complexes containing c-Rel but not p65/RelA (data not shown).
Figure 1.
Identification of protein–DNA complexes formed with the minimal binding sequence from VLTRE after TPA, TNFα, and UVB stimulation. Nuclear extracts were prepared 5 min after UVB irradiation (30 mJ/cm2), 1 h after TNFα treatment (10 ng/ml), and1 h after TPA treatment (100 ng/ml, Pharmacia). The positions of the TPA-, UVB-, and TNFα-induced complexes are labeled 1–3.
TNFα Induces Rel-Dependent Transcription via a PKC-Independent Pathway Similar to UVB.
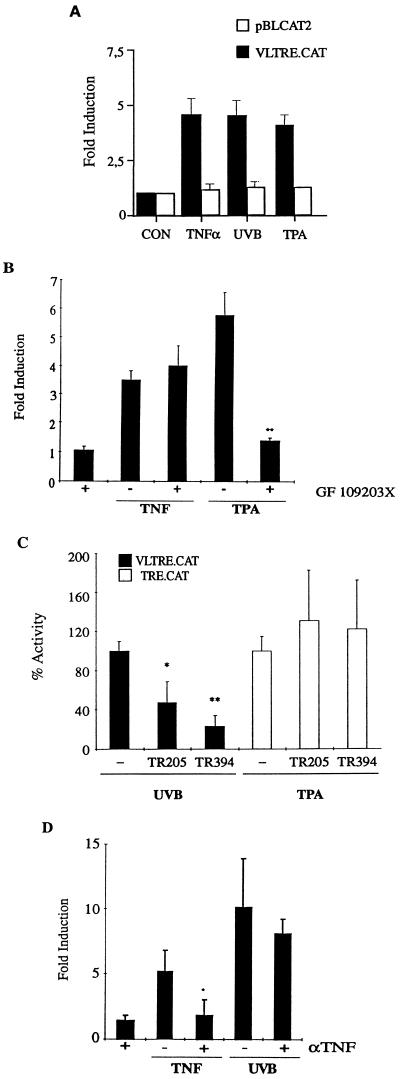
To determine the effect of these stimuli on transcription, we performed transient transfection assays using a VLTRE-driven chloramphenicol acetyltransferase (CAT) reporter plasmid (VLTRE.CAT). The experiments show that TNFα, UVB, and TPA treatment resulted in 4.6-, 4.6-, and 4.1-fold induction, respectively (Fig. 2A). Earlier experiments have shown that TPA- but not UVB-induced VLTRE.CAT activity can be blocked by the PKC inhibitor GF109203X (7). To determine the role of PKC in TNFα-stimulated gene induction, cells were preincubated with 5 μM GF109203X, which inhibited TPA-induced VLTRE.CAT activity but had no inhibitory effect on TNFα-induced VLTRE.CAT activity (Fig. 2B), suggesting that UVB and TNFα may share common signaling pathways.
Figure 2.
(A) Effect of UVB, TNFα, and TPA treatment on Rel-dependent transcription. Preconfluent Balb/MK cells were transiently transfected with 3 μg of reporter plasmid, VLTRE.CAT, or the enhancerless plasmid pBLCAT2. Cells were treated with 10 ng/ml TNFα, 100 ng/ml TPA (Pharmacia), or UVB irradiated (30 mJ/cm2) or untreated (CON) as indicated. 16 h after treatment, cells were assayed for CAT activity. CAT activities were normalized to the activity of a cotransfected Rous sarcoma virus-driven luciferase reporter plasmid. (B) Effect of the PKC inhibitor GF 109203X on TNFα-induced transcription .Transfected cells were treated with 5 μM GF109203X concurrently with 100 ng/ml TPA or 10 ng/ml TNFα as indicated. (C) Inhibitory effect of dominant negative acting TNF receptors on UVB-induced transcription. Balb/MK cells were transiently cotransfected with 3 μg of reporter plasmid, VLTRE.CAT, and 4 μg of dominant negative TNF receptor expression vectors TR 205 and TR 394 (18). Specificity of the TR 294 and TR 394 expression plasmids was tested by assessing the activity of the mutant receptor with an AP-1-responsive reporter plasmid TRE-CAT (3 × AP-1 sites). UVB irradiation resulted in an average 6-fold induction in VLTRE.CAT activity. (D) Effect of anti-TNFα antibody on UVB-induced transcription. Cells were transfected as described, and 5 μl of IP-400 anti-TNFα antibody (Genzyme) was added prior to treatment with either 20 ng/ml TNFα or 30 mJ/cm2 UVB, as indicated. For all experiments, error bars indicate standard error of mean from a minimum of four individual experiments. Statistical analysis was performed using the Student’s t test. ∗, P < 0.05; ∗∗, P < 0.01.
UVB Acts Directly Through the TNF Receptor 1 to Elicit Rel Protein Activity.
To determine whether the TNF receptor has a functional role in UVB-induced Rel activity, we cotransfected VLTRE.CAT with expression vectors for two different forms of dominant negative TNF receptor 1 (Fig. 2C). TR 205 expresses a truncated TNF receptor lacking amino acids 0–204, resulting in the total loss of the cytoplasmic domain and hence loss of all signaling potential (18). TR 394 encodes a C-terminal truncated protein that is unable to stimulate Rel/NF-κB activity but retains the ability to stimulate Raf activity (18, 19). Both forms of mutant receptor caused inhibition of UVB-induced CAT activity but had no effect on a TPA-responsive reporter plasmid. The inhibition of VLTRE.CAT activity by 75% (TR 394) suggests that signaling via the TNF receptor plays a major role in UVB-induced Rel/NF-κB activation in keratinocytes.
One possible explanation for the role of the TNF receptor 1 in mediating UVB effects is that the receptor is being stimulated by its natural ligand. UV irradiation has been shown to induce transcription of the TNFα gene and to cause release of preformed TNFα (20), which can act in an autocrine loop activating NF-κB (21). To examine this possibility, we performed transient transfections in the presence of a neutralizing anti-TNFα antibody (Fig. 2D). TNFα- but not UVB-induced CAT activity was inhibited by an anti-TNFα antibody, suggesting that UVB acts directly on the TNF receptor.
TNF Receptor 1 Associates with Its Downstream Effector, TRAF-2, Following UVB Irradiation.
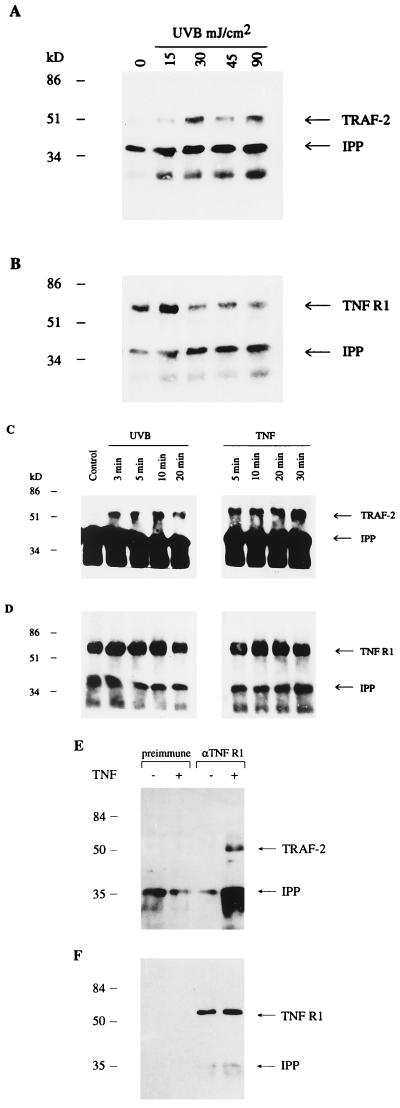
A number of studies have identified TRAF-2 as a key mediator of NF-κB activation by TNF receptors 1 and 2 and CD40 (22, 23). TRAF-2 associates with TNF receptor 1 indirectly via the receptor binding protein TRADD. We performed immunoprecipitation studies using Balb/MK keratinocytes to determine whether TRAF-2 associates with TNF receptor 1 following UVB irradiation. Fig. 3A shows that exposure as low as 15 mJ/cm2 UVB results in detectable TNF receptor–TRAF-2 association with increasing association at higher doses. Weak association of TNF receptor–TRAF-2 is seen at a UV dose that is unable to induce VLTRE.CAT activity. One explanation for this result is that the TNF receptor–TRAF-2 association seen experimentally is below the threshold required for detectable transcription; another explanation is that other UVB-induced pathways are required for full transcriptional activity. For example, the synergistic effect of UV-induced IL-1-, TNF-, and EGF- receptor activity has been proposed to mediate UVB activation of the SAPK/JNK proteins (10). However, in Balb/MK keratinocytes, Ras and Raf do not mediate UVB activation of Rel/NF-κB, and neither EGF nor IL-1 alone are able to induce VLTRE.CAT activity (7) (data not shown).
Figure 3.
UVB irradiation causes a dose-related association of the TNF receptor with TRAF-2. (A) Western blotting performed on TNF receptor-immunoprecipitated extracts using TRAF-2 antibody. The arrows indicate TRAF-2 and the cross-reacting immunoprecipitating antibody (IPP). (B) Following Western blotting for TRAF-2, membranes were stripped, and Western analysis was performed using an antibody (anti-hTNF-BP1, R & D Systems) directed against the TNF receptor (TNF R1) as a means of assessing equality of loading. (C) UVB irradiation causes rapid association of the TNF receptor with TRAF-2. Balb/MK cells were irradiated with 30 mJ/cm2 UVB or treated with 10 ng/ml TNFα, and protein extraction was performed at the time points indicated. Western blotting was performed for TRAF-2 on TNF receptor-immunoprecipitated extracts. The arrows indicate TRAF-2 and the cross-reacting immunoprecipitating antibody (IPP). (D) Following Western blotting for TRAF-2, membranes were stripped, and Western analysis for TNF receptor was performed as a means of assessing equality of loading. (E) To verify the specificity of the immunoprecipitating antibody, immunoprecipitations using preimmune serum or αTNF R1 antibody were performed in parallel. Coimmunoprecipitating TRAF-2 is only detected after TNFα treatment using the αTNF R1 antibody. (F) Following Western blotting for TRAF-2, membranes were stripped, and Western analysis for TNF receptor 1 was performed to verify the specificity of the immunoprecipitating antibody and as a means of assessing equality of loading.
The induction of protein–DNA complexes in UVB-irradiated cells are seen within 5 min by EMSA analysis. Consequently, if TNF receptor–TRAF-2 association is important in the UVB induction of Rel proteins, then this association must be rapid. To test for this association, we performed coimmunoprecipitation studies on cells at different times following UVB irradiation. Fig. 3C shows that TRAF-2 coimmunoprecipitates with TNF receptor 1 within 3 min of UVB irradiation, supporting the role of TNF receptor 1 in the UVB-activated signaling pathway. Similar results were obtained using primary human keratinocytes (data not shown). To verify the specificity of the immunoprecipitating antibody, immunoprecipitations using preimmune serum or αTNF R1 antibody were performed in parallel (Fig. 3 E and F).
Dominant Negative TRAF-2 Inhibits UVB-Induced Rel Protein Activity.
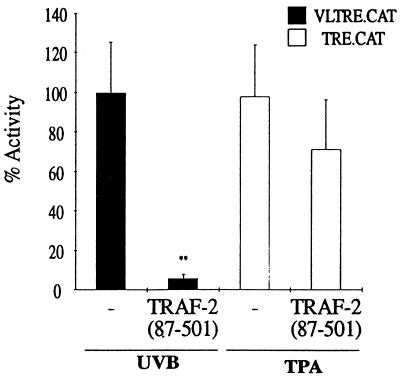
To further investigate the functional significance of the TNF receptor–TRAF-2 association, we cotransfected a TRAF-2 dominant negative plasmid, TRAF-2 (87–501), with VLTRE.CAT and exposed the cells to UVB. This truncated variant of TRAF-2 lacks the RING finger domain, resulting in specific inhibition of TNFα-induced NF-κB activity (24). Fig. 4 shows that TRAF-2 (87–501) causes a 90% decrease in UVB-induced VLTRE.CAT activity, whereas no effect on a TPA-responsive element was seen. This dramatic inhibition of Rel/NF-κB activity suggests that TRAF-2 may constitute a signaling component common to several upstream UVB-induced activators. In comparison, activation of SAPK/JNK seems only partially dependent on TNF receptor–TRAF-2 signaling (10, 25).
Figure 4.
Inhibitory effect of a dominant negative acting TRAF-2 on UVB-induced transcription. Preconfluent Balb/MK cells were transiently cotransfected with 3 μg of reporter plasmid and 4 μg of dominant negative TRAF-2 expression vector TRAF-2 (87–501). Error bars indicate standard error of the mean from a minimum of four individual experiments. Statistical analysis was performed using the Student’s t test; ∗∗, P < 0.01.
DISCUSSION
The skin is the natural target organ for irradiation by UV. Following irradiation, there is an increase in the transcription of genes encoding proteins involved in inflammation and tissue remodeling, such as interleukins, collagenase, and stromelysin. The induction of the transcription factors AP-1 and Rel/NF-κB by UV have been intensely studied because they are important in a cell’s ability to respond to stress and are major contributory factors in inflammation and possibly in immune suppression (1). Moreover, recent results showing that Rel/NF-κB induction can inhibit apoptosis (26–28) implicate UV activation of Rel/NF-κB as a protective part of the mammalian UV response. Understanding how UV regulates NF-κB activity is therefore essential in the study of the effects of UV light on skin. We have shown previously that keratinocytes utilize a Ras-independent pathway to mediate UVB activation of NF-κB proteins. This pathway may be cell type specific because earlier studies using nonepidermal cell types show Ras to be an important component in the pathway mediating UVC activation of NF-κB (29). It is therefore important when considering the photobiology of skin that keratinocytes are used experimentally. Furthermore, the commonly used and biologically potent UVC waveband has not been used in this study, as it is not present in sunlight at ground level; instead we have used the biologically and physiologically important UVB waveband.
We now demonstrate that the kinetics and characteristics of TNFα-induced Rel/NF-κB protein activity and UVB-induced Rel/NF-κB protein activity in keratinocytes are similar. In comparison, TPA induces a different subset of Rel proteins with different kinetics and via different signaling pathway components. Genetic evidence for the role of TNF receptor 1 is provided by the inhibition of UVB-induced Rel-driven transcription by expression of a dominant negative form of TNF receptor 1. Interestingly, inhibition was seen with both the fully truncated TNF receptor and with the mutant receptor T394, which retains the ability to stimulate Raf kinase. Earlier studies have implicated Raf activity in the pathway mediating UVB-induced NF-κB activity; however, we have shown previously that in keratinocytes induced Raf activity does not seem to be important. Thus, the result obtained with T394 further supports the lack of a direct role of Raf in UVB-activated Rel/NF-κB signaling.
UVB irradiation elicits release of TNFα (21), making it likely that the ligand will play a role in activation of the TNF receptor. However, in the present study we found no role for the ligand, TNFα, in UVB-induced transcription. The results therefore imply that a putative autocrine stimulation is not a rapid event, and it can be postulated that TNF receptor activity following UVB irradiation is bimodal, first resulting from the direct action of UVB and second from the UV-induced release of TNFα. Similar conclusions were reached recently by Strickland et al. (30) analyzing TNFα accumulation in human skin in vivo following UVB exposure.
The downstream proteins mediating Rel/NF-κB activation by the TNF receptor are termed TRADD and TRAF proteins. Here, we present biochemical evidence that UVB irradiation of keratinocytes leads to association of endogenous TRAF-2 with the TNF receptor 1 with kinetics in concordance with its role as a mediator of the UVB stimulus. TRAF-2 is known to interact indirectly with TNF receptor 1 via association to TRADD and to mediate activation of Rel/NF-κB in response to TNFα (13). Consistent with this and a recent study by Shu et al. (31), we find that treatment of keratinocytes with TNFα also results in rapid association of endogenous TNF receptor 1 and TRAF-2. Furthermore, expression of a dominant negative version of TRAF-2 leads to almost total inhibition of the UVB-induced Rel/NF-κB-mediated transcriptional activation. This high level of inhibition, which is higher than the corresponding inhibition by dominant negative versions of the TNF receptor 1, suggest that TRAF-2 may be a point of convergence for several upstream activators. Keratinocytes are known to express TNF receptor 1 and CD40 but not TNF receptor 2 (14). However, based on the marked inhibition (75%) achieved also by cotransfecting a dominant negative TNF receptor 1, we suggest that ligand-independent activation of this receptor is of major importance for the UVB-induced Rel/NF-κB activation in keratinocytes. The availability of mice with a targeted disruption of the TNF receptor 1 gene now makes this a testable hypothesis (32).
How TRAF-2 activates downstream components such as IκB kinase(s) is at present unknown. One possibility is that the tyrosine kinase activity required for UVB induction of Rel/NF-κB activity (7) is involved in this process, although this could also be an event upstream of TRAF-2.
A critical role for TRAF-2 in the cellular response to UV irradiation is further indicated by the ability of TRAF-2 to signal JNK/SAPK activation in 293, MCF-7, and COS cells (24, 25, 33). Moreover, TRAF-2 has been shown recently to interact with other proteins, I-TRAF and A20 (34, 35), both of which inhibit Rel/NF-κB activation by TRAF-2 and, in the case of A20, AP-1 activation (36).
Given the ability of TNF receptor 1 to activate apoptosis via a TRADD–FADD interaction and to protect against apoptosis by signaling Rel/NF-κB activation via a TRADD–TRAF-2 interaction, we are faced with a situation where an intricate balance between proapoptotic and protecting signal transducers determines the fate of a cell exposed to UV irradiation. This could also have important implications for the carcinogenic response to UVB. Eliciting Rel/NF-κB activity, protecting against apoptosis, may not be desirable in an irradiated cell because the function of apoptosis is to remove cells deemed to have undergone irreparable damage to the genome.
Thus, a potentially tumorigenic cell, harboring UV-induced mutations, may avoid apoptosis and remain as a target for further genetic changes.
In summary, our results demonstrate biochemically and genetically that UVB can act in keratinocytes via TNF receptor 1 and TRAF-2 to elicit Rel protein–DNA binding, resulting in enhanced transcription. The signaling induced is ligand independent and is likely to be a major pathway for Rel/NF-κB activation by UVB in the skin.
Acknowledgments
We thank Dr. Mats Nilsson for critical reading of the manuscript. This work was supported by grants from the Swedish Cancer Fund.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: TNF, tumor necrosis factor; TNFR, TNF receptor; TRAF, TNF receptor-associated factor; TRADD, TNFR1-associated death domain protein; FADD, Fas-associated death domain protein; PKC, protein kinase C; EGF, epidermal growth factor; IL-1, interleukin 1; CAT, chloramphenicol acetyltransferase; TPA, phorbol 12-tetradecanoate 13-acetate.
References
- 1.Fisher G J, Datta S C, Talwar H S, Wang Z Q, Varani J, Kang S, Voorhees J J. Nature (London) 1996;379:335–339. doi: 10.1038/379335a0. [DOI] [PubMed] [Google Scholar]
- 2.Herrlich P, Ponta H, Rahmsdorf H J. Rev Physiol Biochem Pharmacol. 1992;119:187–223. doi: 10.1007/3540551921_7. [DOI] [PubMed] [Google Scholar]
- 3.Holbrook N J, Fornace A., Jr New Biol. 1991;3:825–833. [PubMed] [Google Scholar]
- 4.Liou H C, Baltimore D. Curr Opin Cell Biol. 1993;5:477–487. doi: 10.1016/0955-0674(93)90014-h. [DOI] [PubMed] [Google Scholar]
- 5.Thanos D, Maniatis T. Cell. 1995;80:529–532. doi: 10.1016/0092-8674(95)90506-5. [DOI] [PubMed] [Google Scholar]
- 6.Siebenlist U, Franzoso G, Brown K. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 7.Tobin D, Nilsson M, Toftgård R. Oncogene. 1996;12:785–793. [PubMed] [Google Scholar]
- 8.Sachsenmaier C, Radler-Pohl A, Zinck R, Nordheim A, Herrlich P, Rahmsdorf H J. Cell. 1994;78:963–972. doi: 10.1016/0092-8674(94)90272-0. [DOI] [PubMed] [Google Scholar]
- 9.Coffer P J, Burgering B M, Peppelenbosch M P, Bos J L, Kruijer W. Oncogene. 1995;11:561–569. [PubMed] [Google Scholar]
- 10.Rosette C, Karin M. Science. 1996;274:1194–1197. doi: 10.1126/science.274.5290.1194. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, Ma W, Bowden G T, Dong Z. J Biol Chem. 1996;271:31262–31268. doi: 10.1074/jbc.271.49.31262. [DOI] [PubMed] [Google Scholar]
- 12.Rothe M, Sarma V, Dixit V M, Goeddel D V. Science. 1995;269:1424–1427. doi: 10.1126/science.7544915. [DOI] [PubMed] [Google Scholar]
- 13.Hsu H, Shu H B, Pan M G, Goeddel D V. Cell. 1996;84:299–308. doi: 10.1016/s0092-8674(00)80984-8. [DOI] [PubMed] [Google Scholar]
- 14.Kristensen M, Chu C Q, Eedy D J, Feldmann M, Brennan F M, Breathnach S M. Clin Exp Immunol. 1993;94:354–362. doi: 10.1111/j.1365-2249.1993.tb03457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrell R. BioEssays. 1996;18:139–148. doi: 10.1002/bies.950180210. [DOI] [PubMed] [Google Scholar]
- 16.Nilsson M, Bohm S. J Virol. 1994;68:276–288. doi: 10.1128/jvi.68.1.276-288.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Struhl K. In: Current Protocols in Molecular Biology. Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Strihl K, editors. New York: Wiley; 1990. pp. 12.0.1–12.1.9. [Google Scholar]
- 18.Wiegmann K, Schutze S, Machleidt T, Witte D, Kronke M. Cell. 1994;78:1005–1015. doi: 10.1016/0092-8674(94)90275-5. [DOI] [PubMed] [Google Scholar]
- 19.Belka C, Wiegmann K, Adam D, Holland R, Neuloh M, Herrmann F, Kronke M, Brach M A. EMBO J. 1995;14:1156–1165. doi: 10.1002/j.1460-2075.1995.tb07099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bazzoni F, Kruys V, Shakhov A, Jongeneel C V, Beutler B. J Clin Invest. 1994;93:56–62. doi: 10.1172/JCI116984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trefzer U, Brockhaus M, Lotscher H, Parlow F, Budnik A, Grewe M, Christoph H, Kapp A, Schopf E, Luger T A, et al. J Clin Invest. 1993;92:462–470. doi: 10.1172/JCI116589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rothe M, Pan M G, Henzel W J, Ayres T M, Goeddel D V. Cell. 1995;83:1243–1252. doi: 10.1016/0092-8674(95)90149-3. [DOI] [PubMed] [Google Scholar]
- 23.Rothe M, Wong S C, Henzel W J, Goeddel D V. Cell. 1994;78:681–692. doi: 10.1016/0092-8674(94)90532-0. [DOI] [PubMed] [Google Scholar]
- 24.Liu Z G, Hsu H, Goeddel D V, Karin M. Cell. 1996;87:565–576. doi: 10.1016/s0092-8674(00)81375-6. [DOI] [PubMed] [Google Scholar]
- 25.Natoli G, Costanzo A, Ianni A, Templeton D J, Woodgett J R, Balsano C, Levrero M. Science. 1997;275:200–203. doi: 10.1126/science.275.5297.200. [DOI] [PubMed] [Google Scholar]
- 26.Van Antwerp D J, Martin S J, Kafri T, Green D R, Verma I M. Science. 1996;274:787–789. doi: 10.1126/science.274.5288.787. [DOI] [PubMed] [Google Scholar]
- 27.Wang C Y, Mayo M W, Baldwin A S., Jr Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- 28.Beg A A, Baltimore D. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- 29.Devary Y, Rosette C, DiDonato J A, Karin M. Science. 1993;261:1442–1445. doi: 10.1126/science.8367725. [DOI] [PubMed] [Google Scholar]
- 30.Strickland I, Rhodes L E, Flanagan B F, Friedmann P S. J Invest Dermatol. 1997;108:763–768. doi: 10.1111/1523-1747.ep12292156. [DOI] [PubMed] [Google Scholar]
- 31.Shu H B, Takeuchi M, Goeddel D V. Proc Natl Acad Sci USA. 1996;93:13973–13978. doi: 10.1073/pnas.93.24.13973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfeffer K, Matsuyama T, Kundig T M, Wakeham A, Kishihara K, Shahinian A, Wiegmann K, Ohashi P S, Kronke M, Mak T W. Cell. 1993;73:457–467. doi: 10.1016/0092-8674(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 33.Reinhard C, Shamoon B, Shyamala V, Williams L T. EMBO J. 1997;16:1080–1092. doi: 10.1093/emboj/16.5.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rothe M, Xiong J, Shu H B, Williamson K, Goddard A, Goeddel D V. Proc Natl Acad Sci USA. 1996;93:8241–8246. doi: 10.1073/pnas.93.16.8241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Song H Y, Rothe M, Goeddel D V. Proc Natl Acad Sci USA. 1996;93:6721–6725. doi: 10.1073/pnas.93.13.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jaattela M, Mouritzen H, Elling F, Bastholm L. J Immunol. 1996;156:1166–1173. [PubMed] [Google Scholar]