Abstract
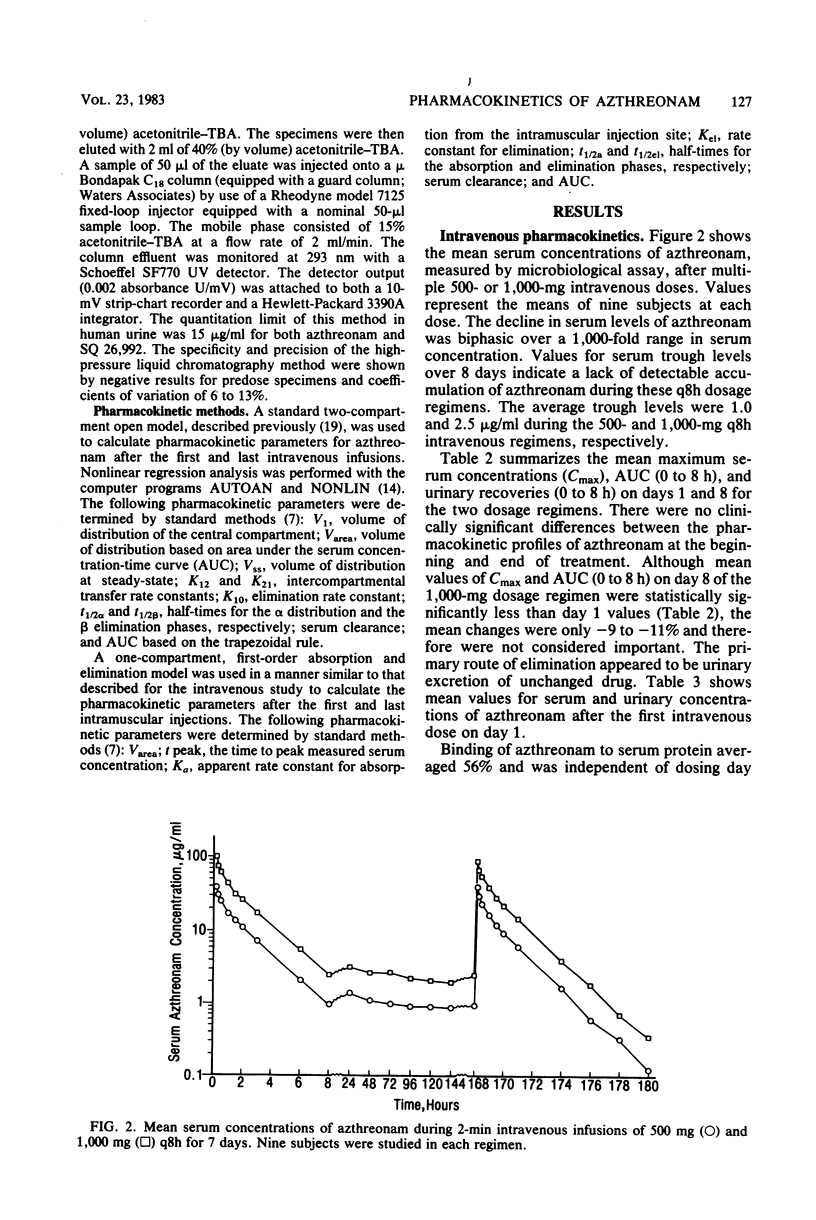
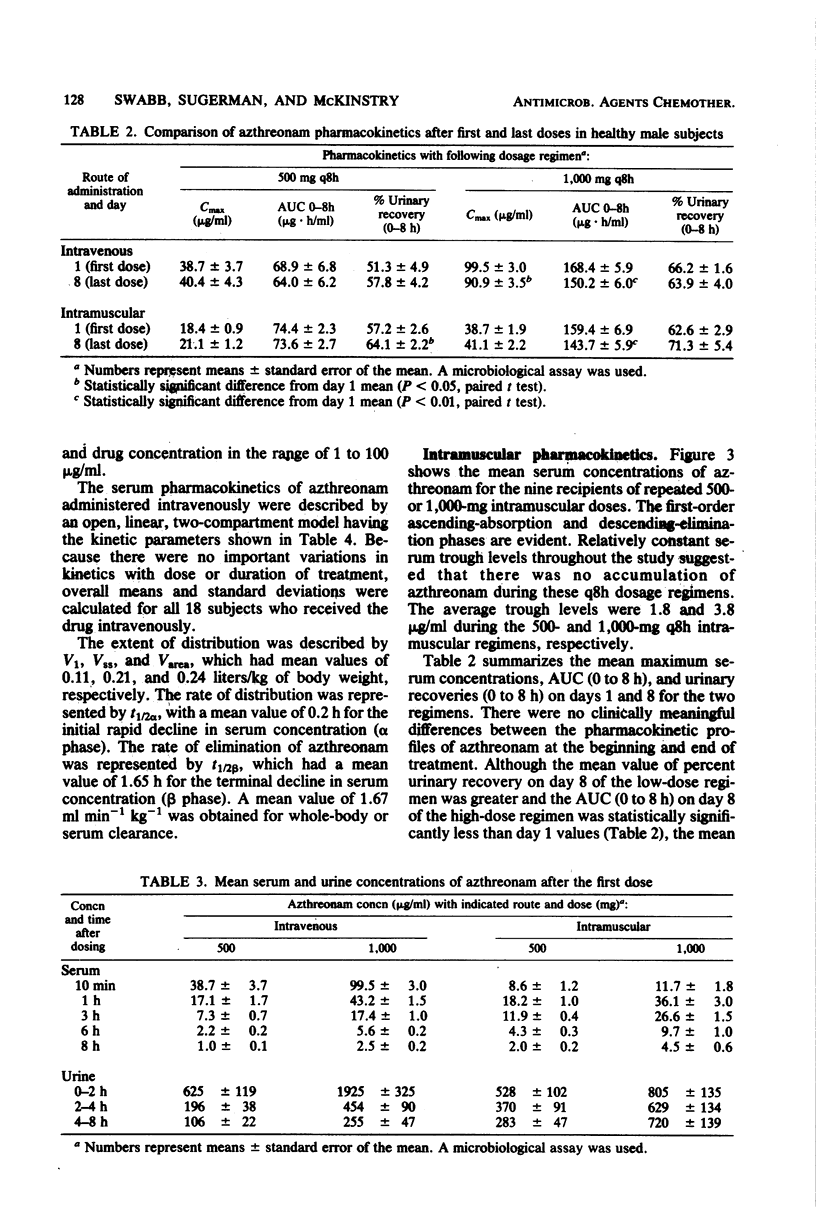
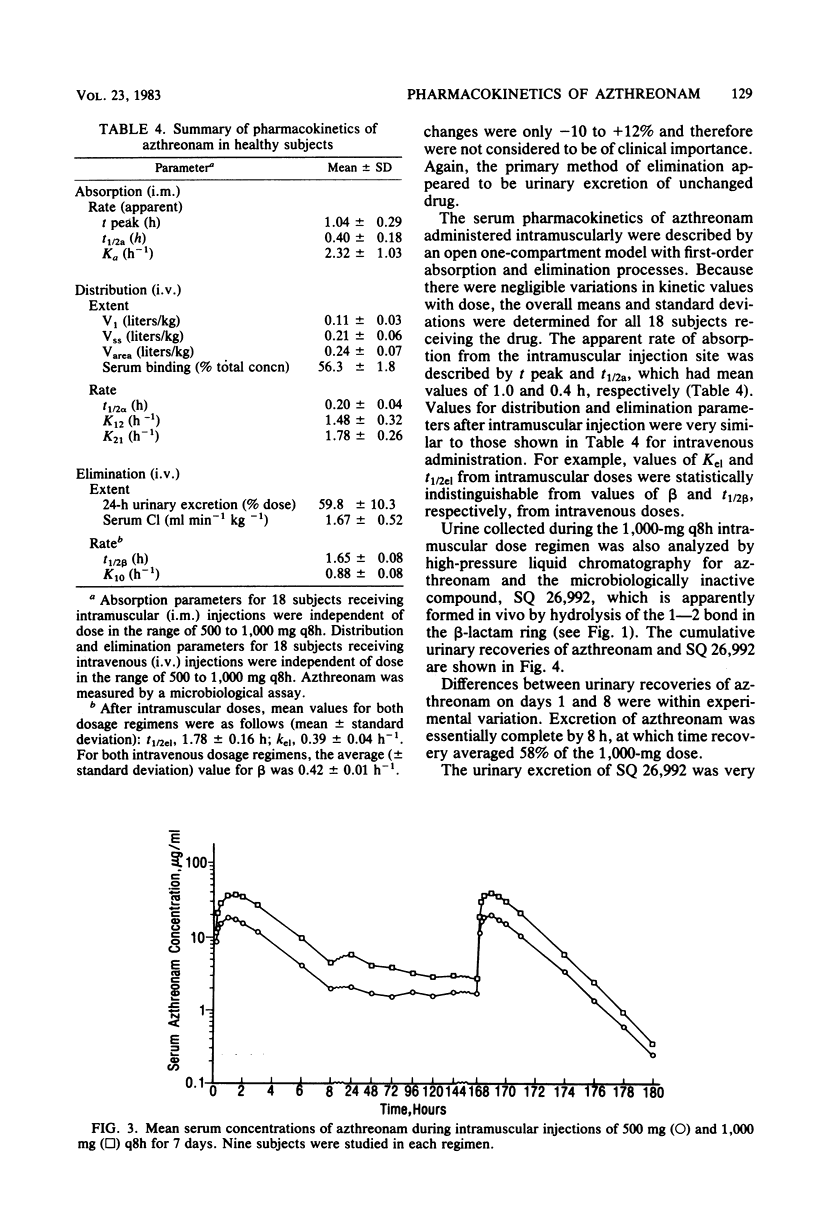
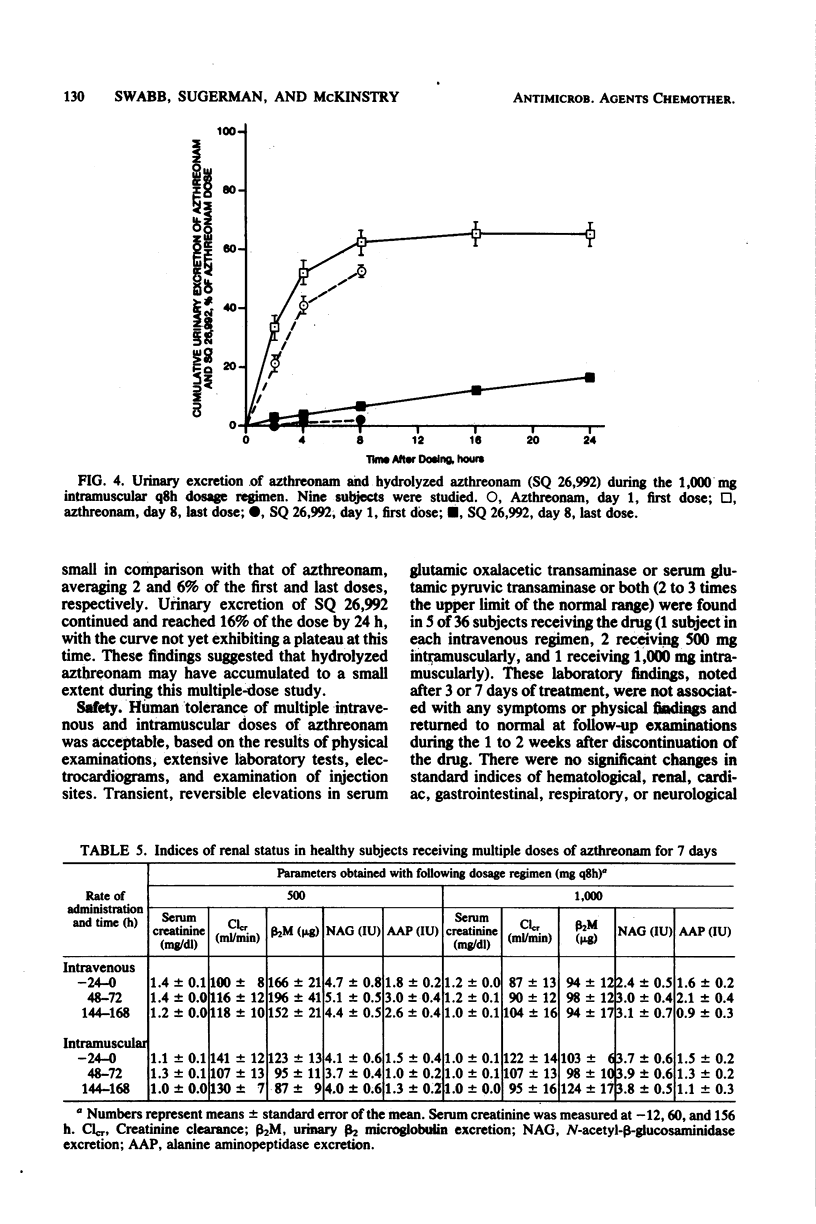
Azthreonam, a monocyclic beta-lactam highly active against aerobic gram-negative bacteria in vitro, was administered to four groups of nine healthy male volunteers in the following four regimens: 500 mg intravenously (i.v.) over 2 min every 8 h, 1,000 mg i.v. over 2 min every 8 h, 500 mg intramuscularly (i.m.) every 8 h, and 1,000 mg i.m. every 8 h for 7 days. Serial samples of serum and urine were assayed by microbiological methods, and urine samples collected during the high-dose i.m. regimen were assayed by a high-pressure liquid chromatographic procedure. Mean peak serum levels were 39 and 99 micrograms/ml after the initial i.v. doses and 18 and 39 micrograms/ml after the initial i.m. doses. There was no evidence of drug accumulation. Mean serum trough levels were 1.0 and 2.5 micrograms/ml during the two i.v. regimens and 1.8 and 3.8 micrograms/ml during the two i.m. regimens. The mean difference among Cmax, area under the curve from 0 to 8 h, and urinary recovery at 0 to 8 h on days 1 and 8 was less than 13% for each regimen. From the i.v. study, the mean steady-state volume of distribution was 0.21 liter/kg, the terminal half-life was 1.6 h, serum clearance was 1.7 ml min-1 kg-1, urinary recovery was 60%, and serum protein binding was 56%. An average of about 6% of a 1,000-mg i.m. dose was excreted in the 8-h urine collection on day 8 as the open beta-lactam ring hydrolysis product of azthreonam. The concentrations of azthreonam in serum were within the range required to inhibit growth of susceptible organisms in vitro.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beam T. R., Jr The third generation of cephalosporins, Part II. Ration Drug Ther. 1982 Jul;16(7):1–5. [PubMed] [Google Scholar]
- Chamberlain J., Coombes J. D., Dell D., Fromson J. M., Ings R. J., Macdonald C. M., McEwen J. Metabolism of cefotaxime in animals and man. J Antimicrob Chemother. 1980 Sep;6 (Suppl A):69–78. doi: 10.1093/jac/6.suppl_a.69. [DOI] [PubMed] [Google Scholar]
- Cole M., Kenig M. D., Hewitt V. A. Metabolism of penicillins to penicilloic acids and 6-aminopenicillanic acid in man and its significance in assessing penicillin absorption. Antimicrob Agents Chemother. 1973 Apr;3(4):463–468. doi: 10.1128/aac.3.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener U., Knoll E., Langer B., Rautenstrauch H., Ratge D., Wisser H. Urinary excretion of N-acetyl-beta-D-glucosaminidase and alanine aminopeptidase in patients receiving amikacin or cis-platinum. Clin Chim Acta. 1981 May 5;112(2):149–157. doi: 10.1016/0009-8981(81)90373-9. [DOI] [PubMed] [Google Scholar]
- Keim G. R., Sibley P. L., Hines F. A., Miller M. M., Peterson A. E., Yoon Y. H. Parenteral toxicological profile of the monocyclic beta-lactam antibiotic SQ 26,776 in mice, rats and dogs. J Antimicrob Chemother. 1981 Dec;8 (Suppl E):141–146. doi: 10.1093/jac/8.suppl_e.141. [DOI] [PubMed] [Google Scholar]
- Lea A. S., Sudan A. W., Wood B. A., Gentry L. O. Randomized comparative study of moxalactam and cefazolin in the treatment of acute urinary tract infections in adults. Antimicrob Agents Chemother. 1982 Jul;22(1):32–35. doi: 10.1128/aac.22.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingston W. K., Elliott A. M., Dismukes W. E., Avent C. K., Cobbs C. G. Clinical evaluation of moxalactam. Antimicrob Agents Chemother. 1981 Jul;20(1):88–97. doi: 10.1128/aac.20.1.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz B., Mogabgab W., Holmes B., Pollock B., Beville R. Clinical evaluation of the therapeutic efficacy and tolerability of piperacillin. Antimicrob Agents Chemother. 1982 Jul;22(1):10–14. doi: 10.1128/aac.22.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondorf A. W., Zegelman M., Klose J., Hendus J., Breier J. Comparative studies on the action of aminoglycosides and cephalosporins on the proximal tubule of the human kidney. J Antimicrob Chemother. 1978 May;4 (Suppl A):53–57. doi: 10.1093/jac/4.suppl_a.53. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Labthavikul P. Antibacterial activity of a monocyclic beta-lactam SQ 26,776. J Antimicrob Chemother. 1981 Dec;8 (Suppl E):111–122. doi: 10.1093/jac/8.suppl_e.111. [DOI] [PubMed] [Google Scholar]
- Nightingale C. H., Greene D. S., Quintiliani R. Pharmacokinetics and clinical use of cephalosporin antibiotics. J Pharm Sci. 1975 Dec;64(12):1899–1926. doi: 10.1002/jps.2600641202. [DOI] [PubMed] [Google Scholar]
- Schentag J. J., Plaut M. E. Patterns of urinary beta 2-microglobulin excretion by patients treated with aminoglycosides. Kidney Int. 1980 May;17(5):654–661. doi: 10.1038/ki.1980.77. [DOI] [PubMed] [Google Scholar]
- Swabb E. A., Leitz M. A., Pilkiewicz F. G., Sugerman A. A. Pharmacokinetics of the monobactam SQ 26,776 after single intravenous doses in healthy subjects. J Antimicrob Chemother. 1981 Dec;8 (Suppl E):131–140. doi: 10.1093/jac/8.suppl_e.131. [DOI] [PubMed] [Google Scholar]
- Swabb E. A., Sugerman A. A., Platt T. B., Pilkiewicz F. G., Frantz M. Single-dose pharmacokinetics of the monobactam azthreonam (SQ 26,776) in healthy subjects. Antimicrob Agents Chemother. 1982 Jun;21(6):944–949. doi: 10.1128/aac.21.6.944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. J., Jr, Niefield S. L., Waters S., Waters B., Awe R. J., Wiss K., Martin R. R., Greenberg S. B. Comparative trial of cefonicid and cefamandole in the therapy of community-acquired pneumonia. Antimicrob Agents Chemother. 1982 Feb;21(2):231–235. doi: 10.1128/aac.21.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]



