Abstract
Strong overexpression of anti-Müllerian hormone (AMH) in transgenic mice leads to incomplete fetal virilization and decreased serum testosterone in the adult. Conversely, AMH-deficient mice exhibit Leydig cell hyperplasia. To probe the mechanism of action of AMH on Leydig cell steroidogenesis, we have studied the expression of mRNA for steroidogenic proteins in vivo and in vitro and performed a morphometric analysis of testicular tissue in mice overexpressing the hormone. We show that overexpression of AMH in male transgenic mice blocks the differentiation of Leydig cell precursors. Expression of steroidogenic protein mRNAs, mainly cytochrome P450 17α-hydroxylase/C17–20 lyase (P450c17), is decreased in transgenic mice overexpressing AMH and in AMH-treated purified Leydig cells. In contrast, transgenic mice in whom the AMH locus has been disrupted show increased expression of P450c17. In vitro, but not in vivo, AMH also decreases the expression of the luteinizing hormone receptor. The effect of AMH is explained by the presence of its receptor on Leydig cells. Our results provide insight into the action of AMH as a negative modulator of Leydig cell differentiation and function.
Anti-Müllerian hormone (AMH), also called Müllerian inhibiting substance or factor, is a glycoprotein dimer belonging to the transforming growth factor β (TGF-β) superfamily and expressed by gonadal somatic cells in both sexes (reviewed in refs. 1 and 2). Immature Sertoli cells, whether fetal or prepubertal, produce high amounts of AMH; at puberty, low amounts accumulate in seminal fluid (3, 4). The AMH receptor (AMHRII), a serine/threonine kinase belonging to the family of type II receptors for members of the TGF-β superfamily, is expressed around fetal Müllerian ducts, in granulosa cells, and in Sertoli cells from fetal life to adulthood in rats (5, 6). The prenatal effect of AMH is well documented and includes the regression of Müllerian ducts, the anlagen of the female internal reproductive tract in male fetuses (7); female transgenic mice chronically overexpressing human AMH (hAMH) under the control of the mouse metallothionein-1 promoter (MT-hAMH mice) lack uterus and Fallopian tubes. In contrast, the role of AMH produced by postnatal Sertoli cells is not well understood. The MT-hAMH male transgenics expressing very high levels of human AMH are incompletely masculinized externally and rapidly become infertile (8). Conversely, both AMH- (9) and AMHR-deficient (10) mice exhibit marked Leydig cell hyperplasia, suggesting an effect of AMH on Leydig cells.
MT-hAMH mice with moderate overexpression of AMH are externally normally masculinized but their serum testosterone is significantly decreased in adulthood (11). To further probe the mechanism of action of AMH on Leydig cell steroidogenesis, we have studied the expression of mRNAs for testicular steroidogenic proteins in vivo and in vitro and performed a morphometric analysis of testicular tissue in MT-hAMH mice. We show that Leydig cells express the AMHR and that overexpression of AMH in male transgenic mice blocks the differentiation of Leydig cell precursors and decreases the expression of steroidogenic enzyme mRNAs.
MATERIALS AND METHODS
Animals.
MT-hAMH transgenic mice (8) were genotyped by dot hybridization of DNA that used the species-specific exon 1 of the human AMH gene (12) as a probe. AMH-deficient mice (The Jackson Laboratories) were genotyped by Southern blot after digestion of DNA by EcoRI and EcoRV enzymes as previously described (9). Only homozygous transgenics were used in the study, all at 2 months of age. Testes and adrenals were removed from transgenic mice and their normal littermates after sacrifice in a carbon dioxide atmosphere. One testis and both adrenals were snap-frozen in liquid nitrogen and stored at −80°C until RNA purification. The controlateral testis of MT-hAMH animals was saved for histological analysis.
Testicular Cell Isolation and Culture.
Briefly, testes from 2-month-old normal mice were decapsulated and digested by collagenase 0.1%. After sedimentation, Leydig cells contained in the supernatant were purified on Percoll gradients (13), yielding preparations containing more than 90% Leydig cells as judged by Δ5-3β-hydroxysteroid dehydrogenase (Δ5-3βHSD) staining. No contaminating Sertoli cells were present, as shown by reverse transcriptase–PCR (RT-PCR) for the Sertoli-cell specific follicle-stimulating hormone (FSH) receptor. Sertoli cells contained in the collagenase pellet were further purified as described (14). Purified cells were plated out in 24-well plates (Costar) at a cell density of 500,000 cells/well in Ham/F12 medium (Life Technologies, Gaithersburg, MD) supplemented as described (13). Cells were cultured 3 days in the presence or absence of AMH (10 μg/ml), the first 24 hr in the presence of 0.2% fetal calf serum and thereafter in serum-free medium.
Hormone Assays.
hAMH serum concentration was assayed by an ELISA (15), which does not recognize the mouse hormone. Testosterone and dihydrotestosterone (DHT) were assayed by specific RIA as described (16, 17). Serum luteinizing hormone (LH) was measured by an immunofluorometric assay (18), and FSH measurements were done with the National Institutes of Health rat FSH RIA kit according to the instructions of the supplier. Serum estradiol, not measurable in individual animals because of the large volume of serum required, was assayed by RIA (19) on serum pools, each obtained from six animals. Results were analyzed by Student’s t test after logarithmic transformation.
Probe Preparation.
RT followed by PCR was used to prepare the probes. The primers are shown in Table 1. Five micrograms of total testicular RNA were reverse-transcribed as described (20). A 5-μl aliquot of this reaction product was amplified by PCR as described (21), except that annealing was at 55°C or 60°C depending on the primers. PCR products were purified on 0.8–1.5% Sea plaque GTG agarose gels (FMC) as described (21), and 32P labeling was performed by random priming.
Table 1.
Oligonucleotide primer pairs
| Target mRNA | Species (ref.) | Sequence of primer (5′ → 3′)
|
|
|---|---|---|---|
| Sense | Antisense | ||
| P450scc | Rat (52) | AGGACTTTCCCTGCGCT | GCATCTCGGTAATGTTGG |
| 3β-HSD | Mouse (52) | TGGTGACAGGAGCAGGA | AGGAAGCTCACAGTTTCCA |
| P450c17 | Mouse (52) | GCCTGACAGACATTCTG | TCGTGATGCAGTGCCCAG |
| StAR | Mouse (53) | ACAACCAGGAAGGCTGGAAG | ATGCAGGTGGGGCCGTGTTCA |
| LHR | Mouse (54) | CCAGAGTTGTCAGGGTCGCGC | AGCATCTGGTTCTGGAGTAC |
| AMHRII | Mouse (10) | TCCAGCTGGCATCCTTTTGC | TGACCTCCTTCCTGGATTAC |
| FSHR | Mouse (55) | GCTCACCAAGCTTCGAGTCA | GCCTTAAAATAGACTTGTTGC |
| Actin | Rat (56) | CGTGGGCCGCCCTAGGCACCA | TTGGCCTTAGGGTTCAGAGGGG |
| Ribosomal | Rabbit (57) | CCCGCCACACATGTTTCCCGTCCCT | |
Northern Analysis.
Total mRNA was purified (22) from testes and adrenals of nine animals in each group, and RNA samples were resolved on a 1.2% agarose-1% formaldehyde gel, blotted, and hybridized as described (20). After hybridization, the blots were stripped with 0.1× sodium saline citrate (SSC)/1× SDS at 100°C three times for 10 min and rehybridized with the different probes. A rabbit ribosomal probe (Table 1) was used as a control, and a 0.24- to 9.5-kb RNA ladder (Life Technologies) as size marker. Blots were autoradiographed with Kodak X-Omat film and scanned on a PhosphorImager before semiquantitative analysis with Image-Quant Software (Molecular Dynamics). The densitometric signal was expressed as a percentage of the value obtained for a normal littermate analyzed on the same blot. Results were subjected to Student’s paired t test.
RT-PCR.
RT-PCR was used to study gene expression in purified Leydig cells, because of the low amount of RNA available. At the end of the culture period, total RNAs were extracted from Leydig cells with the RNA Plus kit (Bioprobe Systems, Montreuil-sous-Bois, France) according to the instructions of the manufacturer. The expression of AMHRII, P450 cholesterol side-chain cleavage (P450scc), 3β-HSD, P450 17α-hydroxylase/C17–20 lyase (P450c17), LH receptor (LHR), FSH receptor, steroidogenic acute regulatory protein (StAR), and actin was studied, by using primers shown on Table 1. RT-PCR conditions were as described above except that for AMHRII, FSH receptor, and LHR denaturation was at 94°C for 1 min, annealing at 55°C for 1 min 50 sec, and extension at 72°C for 2 min. To check the specificity of the reaction, 20 μl of the reaction products were removed at 20, 25, 30, 35, and 40 cycles of the PCR. When semiquantification was desired, gels were blotted, hybridized with specific oligonucleotides, and analyzed by PhosphorImaging.
Morphometric Analysis of Testicular Tissue.
Morphometric analysis was performed on one testis of three MT-hAMH mice and three control littermates. As testicular specific gravity is ≈1, testicular weight was used to estimate testicular volume. The whole testis, fixed in Bouin and embedded in paraffin wax, was serially sectioned at 7 μm. The total number of sections was recorded, and one section of 50 was mounted and stained with hematoxylin-eosin for histologic and morphometric studies, as described by Vergouwen et al. (23), with slight modifications. Relative volume (volume density) of testicular components was obtained by using a semiautomatic image analyzer Biocom 200 and Imagenia software (Biocom, Courtaboeuf, France). Absolute volume of testicular components was calculated by multiplying their relative volume by whole testicular volume (24). Mesenchymal as well as immature and mature Leydig cells were identified in the interstitial tissue according to criteria previously described (25, 26). Mature Leydig cells showed a large, rounded nucleus containing a conspicuous nucleolus; immature Leydig cells had a smaller oval-shaped nucleus with heterochromatin granules and less abundant cytoplasm; mesenchymal cells were elongated with scarce cytoplasm. The total number per testis of each cell type was obtained by multiplying by 50 the total number of cells counted in all the slides obtained from one gonad. Results were subjected to a “ratio” paired t test, where the relative, instead of the absolute, difference between paired samples is compared with 0 (27).
RESULTS
MT-hAMH Mice.
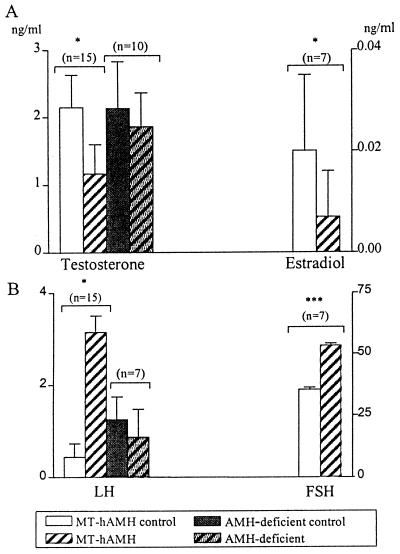
Determination of serum hormone concentrations. Serum levels of hAMH, testosterone, estradiol, LH, and FSH were measured in 7–15 MT-hAMH transgenic mice and their wild-type littermates. All were normally virilized and fertile. Mean hAMH levels were 276.7 ± 52.2 ng/ml in MT-hAMH mice. AMH levels in normal 2-month-old mice, measured by an ELISA recognizing mouse AMH, are approximately 15 ng/ml (28). The mean serum levels of testosterone and estradiol were significantly decreased (Fig. 1A), in spite of high individual variability, as is typical of serum testosterone levels of normal mice (29). Serum LH and FSH were significantly increased by a factor of 8 and 1.5, respectively (Fig. 1B), with high individual variability for LH, probably caused by pulsatile secretion.
Figure 1.
Serum testosterone and estradiol (A) and LH and FSH (B) were measured in MT-hAMH transgenics, AMH-deficient mice, and their respective littermates (control) at 2 months of age. Results represent mean and SEM for the specified number of animals, except for estradiol, where n represents the number of serum pools analyzed. ∗, P ≤ 0.05, ∗∗∗, P ≤ 0.001.
In another series of five transgenic mice and four normal littermates, testosterone (T) and DHT were assayed in serum and testicular tissue. In serum, the mean ± SD T/DHT ratio was 3.59 ± 0.88 in transgenics compared with 6.48 ± 7.14 in littermates (not significant); in testicular tissue, the difference between transgenics (9.76 ± 3.76) and normal littermates (49.08 ± 20.1) was significant (P < 0.01).
Expression of mRNA coding for steroidogenic proteins.
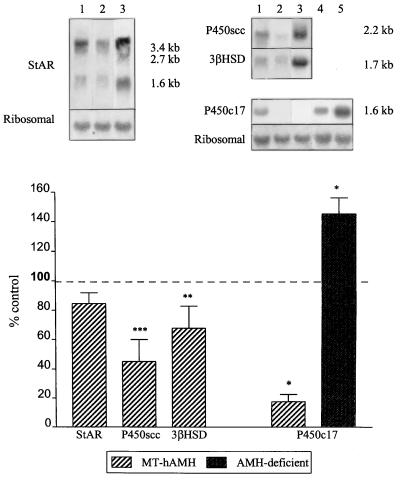
To determine the cause for the decreased serum testosterone concentration in MT-hAMH mice, the mRNAs coding for various proteins involved in testicular steroidogenesis were analyzed. StAR, a mitochondrial phosphoprotein, is involved in the translocation of cholesterol across the mitochondrial membrane (30, 31). The P450scc, a mitochondrial enzyme, and P450c17, which resides in the smooth endoplasmic reticulum, both belong to the cytochrome P450 superfamily. In the mouse 3β-HSD multigene family, type I is the only isoform expressed exclusively in the gonads and adrenals (32). Expression of P450scc, 3β-HSD, and P450c17 mRNAs was significantly decreased in MT-hAMH mice (Fig. 2); P450c17 was the most affected, with an expression only 12% of normal, whereas expression of StAR transcripts did not differ significantly between transgenics and their normal littermates (Fig. 2). Expression of mRNAs coding for steroidogenic factor 1, a regulator of steroidogenic proteins (33) and for tissue inhibitor of metalloproteinases, a Sertoli cell product that contributes to the regulation of Leydig cell steroidogenesis (34) was also normal (results not shown).
Figure 2.
Expression of steroidogenic protein mRNAs in the testes of transgenic animals. (Upper) Northern blot hybridization of StAR (Left) and P450scc, 3β-HSD, and P450c17 probes (Right) to testicular RNA extracted from 1) normal littermate of MT-hAMH transgenic, 2) MT-hAMH transgenic, 3) adrenals of normal mice, 4) normal littermate of AMH-deficient transgenic, and 5) AMH-deficient mouse. Results shown are representative of those obtained for nine MT-hAMH and five AMH-deficient animals and the same number of normal littermates. Total RNA samples were 20 μg for testicular tissue and 5 μg for adrenal; exposure was 4 hr for P450scc, 12 hr for 3β-HSD and P450c17, and 48 hr for StAR. (Lower) PhosphorImaging quantification of steroidogenic proteins mRNA expression in MT-hAMH and AMH-deficient mice. Results are expressed as mean ± SEM (n = 9 for MT-hAMH and n = 5 for AMH-deficient mice) of percentage of the expression relative to a normal littermate (control) analyzed on the same blot. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01; ∗∗∗, P ≤ 0.001.
Expression of LHR mRNAs.
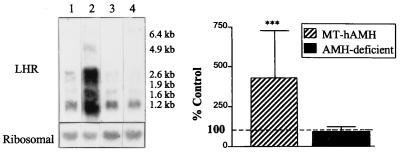
Leydig cell development and function are induced by binding of LH to LHR, a G-protein-coupled receptor located on the Leydig cell membrane (reviewed in ref. 35). Testicular expression of LHR transcripts was increased 5-fold in MT-hAMH mice compared with normal littermates. All splice variants were similarly affected (Fig. 3).
Figure 3.
Expression of LHR mRNA in the testes of transgenic animals. (Left) Northern blot hybridization of LHR probe to testicular RNA. Total RNA samples were 20 μg, exposure was 1 week. 1) normal littermate of MT-hAMH transgenic, 2) MT-hAMH transgenic, 3) normal littermate of AMH-deficient transgenic, and 4) AMH-deficient transgenic. Results shown are representative of those obtained for nine MT-hAMH and five AMH-deficient animals and the same number of normal littermates. (Right) PhosphorImager quantification of LHR transcripts. Results are expressed as mean ± SEM (n = 9 for MT-hAMH and n = 5 for AMH-deficient mice) of percentage of expression relative to a normal littermate (control) analyzed on the same blot. ∗∗∗, P ≤ 0.001.
Morphometric studies.
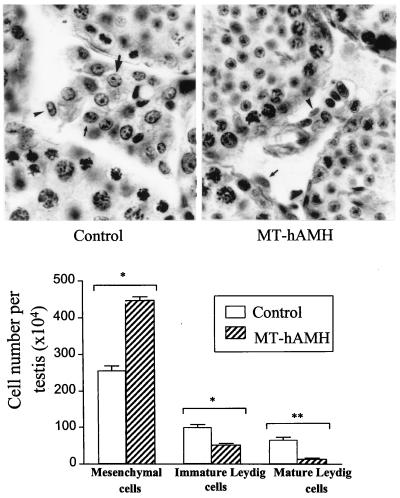
Testicular volume, which in adults is mainly dependent on germ cell number (23, 36), was similar in MT-hAMH mice and their normal littermates (Table 2). Although we did not perform quantitative studies of spermatogenesis, qualitative histological examination (Fig. 4) and morphometric data of seminiferous tubule parameters (Table 2) indicated that spermatogenesis was not affected in transgenic mice. Results of morphometric analysis of interstitial cell populations are shown in Fig. 4. Adult Leydig cells descend from a cohort of mesenchymal cells, which become committed to the Leydig cell lineage through three distinct stages of development: Leydig cell precursors, morphologically indistinguishable from mesenchymal cells, immature and mature Leydig cells (26). In MT-hAMH animals, the number of immature and mature Leydig cells was decreased by 49.7% and 79.8%, respectively, whereas the number of mesenchymal cells, which include Leydig cell precursors, was increased by 73.8% over normal littermates. Altogether, the cumulated number of mesenchymal and Leydig cells was similar in the two groups (511 ± 114 and 416 ± 226 × 104 in MT-hAMH animals and their normal littermates, respectively). No degenerative changes were observed in Leydig cells of transgenic animals.
Table 2.
Morphometric analysis of testicular tissue from MT-hAMH mice and their normal littermates (control)
| Control | MT-hAMH | |
|---|---|---|
| Testicular volume (mm3) | 83.3 ± 9.02 | 81.3 ± 7.8 |
| Total Leydig cell volume (mm3) | 5.4 ± 2.9 | 1.4 ± 0.97* |
| Seminiferous tubule volume (mm3) | 67.6 ± 10.8 | 66.5 ± 7.5 |
| Tubular diameter (μm) | 166.1 ± 9.6 | 170.2 ± 10.2 |
Values are mean ± SEM, n = 3. ∗, P < 0.05, by Student’s t test.
Figure 4.
(Upper) Histological sections of testicular tissue from a 2-month-old MT-hAMH transgenic mouse and its normal littermate (control). Mature Leydig cells (large arrows) show a large, rounded nucleus containing a conspicuous nucleolus; immature Leydig cells (small arrows) have a smaller oval-shaped nucleus with heterochromatin granules and less abundant cytoplasm; mesenchymal cells (arrowheads) are elongated with scarce cytoplasm. Interstitial tissue was more abundant and Leydig cell groups were more conspicuous in normal mice. Hematoxylin-eosin stain, ×700. (Lower) Number (mean ± SEM) of mesenchymal and immature and mature Leydig cells per testis in MT-hAMH mice and their normal littermates (control) n = 3. ∗, P ≤ 0.05; ∗∗, P ≤ 0.01.
AMH-Deficient Mice.
AMH-deficient mice, obtained by gene targeting of the AMH locus (9), are the mirror image of MT-hAMH transgenics. Therefore the parameters most affected by overexpression of AMH also were studied in male AMH-deficient animals. Morphometric analysis was not performed, because Leydig cell hyperplasia is already known to occur (9). Serum testosterone and LH were not significantly different from normal littermates (Fig. 1) but Northern blot hybridization of testicular RNA showed 45% increased expression of P450c17 (Fig. 2). LHR expression was not significantly different from controls (Fig. 3).
Purified Leydig Cells.
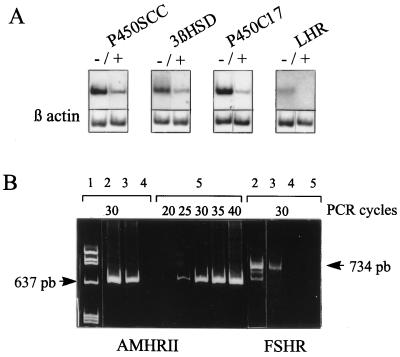
To determine whether the effect of AMH on steroidogenesis was caused by a direct effect on Leydig cells or was mediated by Sertoli cells, we analyzed by RT-PCR the effect of AMH on Leydig cells isolated from 2-month-old normal mice. AMH decreased the expression of steroidogenic enzymes mRNAs and LHR transcripts (Fig. 5A) but had no effect on StAR expression (results not shown). PhosphorImager quantification, performed in three instances at 30 cycles, showed a mean 58.25 ± 8.1% decrease for P450c17, 41 ± 9.3% for 3β-HSD, 34.3 ± 3.9% for P450scc, and 100% for LHR. An AMHRII transcript was visible in six independent preparations of Leydig cells as early as 25 cycles in Leydig cells (Fig. 5B). It was also present, as expected, in whole testicular tissue and in purified Sertoli cells, but not in spleen. A FSH receptor transcript was visible in purified Sertoli cells but not in the Leydig cell preparations, indicating the absence of contamination.
Figure 5.
Expression studies of various steroidogenic protein mRNAs in purified Leydig cells. (A) Southern blot hybridization of RT-PCR products (30 cycles) of P450c17, 3β-HSD, P450scc, and LHR. RNA was extracted from purified Leydig cells incubated 3 days in the presence (+) or absence (−) of 10 μg/ml of AMH. Actin served as a control. Results shown are representative of three independent experiments. (B) RT-PCR of AMHRII and FSH receptor in different testicular cell types: 1) molecular weight marker, 2) whole testicular tissue, 3) purified Sertoli cells, 4) spleen, all analyzed at 30 cycles, and 5) purified Leydig cells, analyzed at 20 to 40 cycles. Results shown are representative of six independent experiments.
DISCUSSION
The present results demonstrate that AMH exerts an inhibitory effect on Leydig cell differentiation and function. Briefly, differentiation of Leydig cell precursors was blocked in MT-hAMH transgenic mice overexpressing AMH, leading to a decreased number of mature Leydig cells. AMH also repressed steroidogenic enzyme expression both in purified Leydig cells and in MT-hAMH mice testis but had divergent effects on LHR transcripts, depending on the experimental conditions. In MT-hAMH mice, all species of LHR transcripts were up-regulated, whereas addition of AMH to culture medium of purified Leydig cells abolished LHR expression. The AMHR, formerly thought to reside only on the membrane of Sertoli cells, also was detected in Leydig cells where, in all likelihood, it serves to transduce the effect of AMH.
Other members of the transforming growth factor β family have been shown to negatively affect Leydig cell function (reviewed in ref. 37). Transforming growth factor β and activin inhibit steroidogenic response to human chorionic gonadotrophin. Inhibin has no clear effect on steroidogenesis but inhibin-deficient mice develop gonadal Sertoli/granulosa cell tumors (38). Inhibin and AMH exert a synergistic effect on testicular tumorigenesis (39). Our results provide insight into the cellular mechanisms involved in the action of AMH as a repressor of Leydig cell proliferation.
Data from the literature indicate that the postnatal proliferation of Leydig cells is mainly the result of differentiation from mesenchymal cells. The fact that, in adult mammalian testes, only one in 20,000 Leydig cells undergoes mitosis and the inverse relationship between the number of Leydig cells and their mesenchymal precursors in the developing rat (40) suggest that differentiation from mesenchymal cells is the main factor involved in the pubertal proliferation of Leydig cells. Furthermore, in rats injected with the cytotoxic drug ethylene-1,2-dimethane sulfonate, destruction of Leydig cells is followed by a wave of proliferative activity in mesenchymal cells (41). The decreased ratio of Leydig cells versus mesenchymal precursors, whereas their cumulated number is normal, suggests that the Leydig cell hypoplasia observed in MT-hAMH mice is caused by a block in the differentiation of Leydig cell precursors (Fig. 4B). The testosterone/DHT ratio was decreased in MT-hAMH versus normal mice, indicating a higher 5α-reductase activity in the testis of the transgenic mice, in keeping with the high proportion of immature cells (42). The difference was significant in testicular tissue but not in serum because of high individual variability and also because testicular contribution to serum DHT appears relatively minor.
Not all steroidogenic proteins were similarly affected by AMH in vivo. P450c17 was the most sensitive, whereas 3β-HSD and StAR were only marginally affected (Fig. 2). This difference may be related to Leydig cell precursors expressing variable amounts of steroidogenic enzymes (26). Because the cumulated number of precursors and Leydig cells in the interstitial compartment is the same in normal and MT-hAMH transgenic mice, proteins highly expressed by precursors, for instance 3β-HSD, would be less affected by AMH. This explanation is supported by the fact that, in purified Leydig cells of normal mice, AMH exerted a similar effect on the expression of all steroidogenic mRNAs studied (Fig. 5A).
The opposite effect of AMH on the LHR in vitro and in vivo is not easily accounted for. In purified Leydig cells, AMH inhibited the expression of LHR (Fig. 5A); in contrast, testicular LHR mRNA expression was increased in transgenic animals overexpressing AMH (Fig. 3). A specific inhibitory effect of AMH on expression of LHR, also seen in cultured granulosa cells (43), was perhaps obscured in vivo by the LH feedback mechanism. Decreased testosterone production leads to enhanced pituitary secretion of LH, physiological levels of which are known to increase LHR transcription (32, 44). The difference in cell maturity between in vivo and in vitro experiments also should be taken in consideration, in the event that AMH should exert a different effect on mature versus immature Leydig cells, as is known to occur for human chorionic gonadotrophin (45).
Are the effects of AMH on steroidogenesis and Leydig cell differentiation related? Androgens promote differentiation of immature Leydig cells from mesenchymal precursors (26), suggesting that the Leydig cell maturation defect observed in MT-hAMH mice is at least partly caused by the concomitant repression of testosterone production. Conversely, the low number of mature Leydig cells in MT-hAMH mice obviously decreases the efficiency of steroidogenesis in vivo. However, the fact that AMH decreases the expression of steroidogenic enzyme mRNA in cultured Leydig cells proves that AMH can affect Leydig cell function independently of cell number (Fig. 5A). In vivo, this effect is presumably receptor-independent, because it occurs in the presence of increased amounts of LHR; in vitro, however, we cannot rule out the possibility that the inhibition of steroidogenic enzyme mRNA could be related to the low expression of LHR. Identifying the primary effect of AMH on the Leydig cell will not be a simple task, because of the intricate interactions between Leydig cell differentiation, LH and testosterone production, expression of the LH receptor and steroidogenic enzymes (26, 32), all of which are affected by AMH.
Paracrine factors play an important role in modulating Leydig cell function (34, 46). Because reports from several groups (5), including our own (20), had mapped the AMHR to Sertoli cells, we initially assumed that the effect of AMH on Leydig cell function of transgenic mice was mediated through receptors located on Sertoli cells. The finding that AMH decreases transcription of steroidogenic enzymes and LHR in purified Leydig cells led us to reconsider and to take a second look at the sites of expression of AMHR in the testis. Using RT-PCR, we clearly detected expression of the AMHRII mRNA in Leydig cells (Fig. 5B). Expression by both Sertoli and Leydig cells has been described for several proteins, such as inhibin-related peptides (47) and steroidogenic factor-1 (48). The present work demonstrates a paracrine effect of AMH, produced by Sertoli cells, on Leydig cells expressing the AMHR.
Alfred Jost, the discoverer of AMH (7), called it an anti-female hormone, because it inhibits the development of ovaries and internal reproductive tract in the female fetus (49). After birth, although Müllerian derivatives have become resistant to its action, AMH inhibits ovarian follicular maturation (43, 50). Our present results exonerate AMH from sexism (51) by showing that the inhibitory effect of AMH on gonadal maturation also extends to the male.
Acknowledgments
Marie-Pierre Monneret and Tarja Laiho provided expert technical assistance. We are grateful to Dr. Gaston Godeau (Faculté de Chirurgie Dentaire, Montrouge) for the use of the image analyzer and to Dr. Alena Leroux (Unité 129 Institut National de la Santé et de la Recherche Médicale, Institut Cochin de Génétique Moléculaire) for allowing us access to the PhosphorImager at her institution. We are grateful to Dr. Richard Behringer for the gift of MT-hAMH transgenic mice, to Dr. Keith Parker for the gift of the steroidogenic factor 1 probe, and to Dr. José Sáez for helpful advice. R.R. is recipient of a postdoctoral fellowship of the National Research Council (Consejo Nacional de Investigaciones Científicas y Técnicas de Argentina).
ABBREVIATIONS
- AMH
anti-Müllerian hormone
- AMHR
AMH receptor
- hAMH
human AMH
- MT
metallothionein 1
- RT-PCR
reverse transcriptase–PCR
- FSH
follicle-stimulating hormone
- DHT
dihydrotestosterone
- LH
luteinizing hormone
- LHR
LH receptor
- StAR
steroidogenic acute regulatory protein
- HSD
hydroxysteroid dehydrogenase
References
- 1.Josso N, Cate R L, Picard J Y, Vigier B, di Clemente N, Wilson C, Imbeaud S, Pepinsky R B, Guerrier D, Boussin L, Legeai L, Carré-Eusèbe D. In: Recent Progress in Hormone Research. Bardin C W, editor. Vol. 48. San Diego: Academic; 1993. pp. 1–59. [DOI] [PubMed] [Google Scholar]
- 2.Lee M M, Donahoe P K. Endocr Rev. 1993;14:152–164. doi: 10.1210/edrv-14-2-152. [DOI] [PubMed] [Google Scholar]
- 3.Fallat M E, Siow Y, Belker A M, Boyd J K, Yoffe S, MacLaughlin D T. Hum Reprod. 1996;11:2165–2169. doi: 10.1093/oxfordjournals.humrep.a019070. [DOI] [PubMed] [Google Scholar]
- 4.Vigier B, Tran D, du Mesnil du Buisson F, Heyman Y, Josso N. J Reprod Fertil. 1983;69:207–214. doi: 10.1530/jrf.0.0690207. [DOI] [PubMed] [Google Scholar]
- 5.Baarends W M, van Helmond M J L, Post M, van der Schoot P C J M, Hoogerbrugge J W, de Winter J P, Uilenbroek J T J, Karels B, Wilming L G, Meijers J H C, Themmen A P N, Grootegoed J A. Development (Cambridge, UK) 1994;120:189–197. doi: 10.1242/dev.120.1.189. [DOI] [PubMed] [Google Scholar]
- 6.Teixeira J, He W W, Shah P C, Morikawa N, Lee M M, Catlin E A, Hudson P L, Wing J, MacLaughlin D T, Donahoe P K. Endocrinology. 1996;137:160–165. doi: 10.1210/endo.137.1.8536608. [DOI] [PubMed] [Google Scholar]
- 7.Jost A. Recent Progr Horm Res. 1953;8:379–418. [Google Scholar]
- 8.Behringer R R, Cate R L, Froelick G J, Palmiter R D, Brinster R L. Nature (London) 1990;345:167–170. doi: 10.1038/345167a0. [DOI] [PubMed] [Google Scholar]
- 9.Behringer R R, Finegold M J, Cate R L. Cell. 1994;79:415–425. doi: 10.1016/0092-8674(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 10.Mishina Y, Rey R, Finegold M J, Matzuk M M, Josso N, Cate R L, Behringer R R. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- 11.Lyet L, Louis F, Forest M G, Josso N, Behringer R R, Vigier B. Biol Reprod. 1995;52:444–454. doi: 10.1095/biolreprod52.2.444. [DOI] [PubMed] [Google Scholar]
- 12.Cate R L, Mattaliano R J, Hession C, Tizard R, Farber N M, Cheung A, Ninfa E G, Frey A Z, Gash D J, Chow E P, Fisher R A, Bertonis J M, Torres G, Wallner B P, Ramachandran K L, Ragin R C, Manganaro T F, MacLaughlin D T, Donahoe P K. Cell. 1986;45:685–698. doi: 10.1016/0092-8674(86)90783-x. [DOI] [PubMed] [Google Scholar]
- 13.Bernier M, Chatelain P G, Mather J P, Saez J M. J Cell Physiol. 1986;129:257–263. doi: 10.1002/jcp.1041290218. [DOI] [PubMed] [Google Scholar]
- 14.Perrard-Sapori M H, Sáez J M, Dazord A. Mol Cell Endocrinol. 1985;43:189–197. doi: 10.1016/0303-7207(85)90083-8. [DOI] [PubMed] [Google Scholar]
- 15.Carré-Eusèbe D, Imbeaud S, Harbison M, New M I, Josso N, Picard J Y. Hum Genet. 1992;90:389–394. doi: 10.1007/BF00220465. [DOI] [PubMed] [Google Scholar]
- 16.Forest M G, Cathiard A M, Bertrand J. J Clin Endocrinol Metab. 1973;36:1132–1142. doi: 10.1210/jcem-36-6-1132. [DOI] [PubMed] [Google Scholar]
- 17.Sáez J M, Forest M G, Morera A M, Bertrand J. J Clin Invest. 1972;51:1226–1234. doi: 10.1172/JCI106917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haavisto A M, Petterson K, Bergendahl M, Perheentupa A, Roser J F, Huhtaniemi I. Endocrinology. 1993;132:1687–1691. doi: 10.1210/endo.132.4.8462469. [DOI] [PubMed] [Google Scholar]
- 19.Forest M G, de Peretti E, Lecoq A, Cadillon E, Zabot M T, Thoulon J M. J Clin Endocrinol Metab. 1980;51:816–822. doi: 10.1210/jcem-51-4-816. [DOI] [PubMed] [Google Scholar]
- 20.di Clemente N, Wilson C A, Faure E, Boussin L, Carmillo P, Tizard R, Picard J Y, Vigier B, Josso N, Cate R L. Mol Endocrinol. 1994;8:1006–1020. doi: 10.1210/mend.8.8.7997230. [DOI] [PubMed] [Google Scholar]
- 21.Imbeaud S, Faure E, Lamarre I, Mattei M G, di Clemente N, Tizard R, Carré-Eusèbe D, Belville C, Tragethon L, Tonkin C, Nelson J, McAuliffe M, Bidart J M, Lababidi A, Josso N, Cate R L, Picard J Y. Nat Genet. 1995;11:382–388. doi: 10.1038/ng1295-382. [DOI] [PubMed] [Google Scholar]
- 22.Chirgwin J M, Przybyla A E, MacDonald R J, Rutter W J. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- 23.Vergouwen R P F A, Huiskamp R, Bas R J, Roepersgajadien H L, Davids J A G, Derooij D G. J Reprod Fertil. 1993;99:479–485. doi: 10.1530/jrf.0.0990479. [DOI] [PubMed] [Google Scholar]
- 24.Weibel E R, Kistler G S, Scherle W F. J Cell Biol. 1966;30:23–38. doi: 10.1083/jcb.30.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rey R, Nagle C, Chemes H. Tissue Cell. 1996;28:31–42. doi: 10.1016/s0040-8166(96)80042-5. [DOI] [PubMed] [Google Scholar]
- 26.Ge R S, Shan L X, Hardy M P. In: The Leydig Cell. Payne A H, Hardy M P, Russell L D, editors. Vienna, IL: Cache River Press; 1996. pp. 159–173. [Google Scholar]
- 27.Motulsky H. Intuitive Biostatistics. New York: Oxford Univ. Press; 1995. p. 227. [Google Scholar]
- 28.Al-Attar L, Noël K, Dutertre M, Belville C, Forest M G, Burgoyne P S, Josso N, Rey R. J Clin Invest. 1997;100:1335–1343. doi: 10.1172/JCI119653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bartke A, Steele R E, Musto N, Caldwell B V. Endocrinology. 1973;92:1223–1228. doi: 10.1210/endo-92-4-1223. [DOI] [PubMed] [Google Scholar]
- 30.Clark B J, Soo S C, Caron K M, Ikeda Y, Parker K L, Stocco D M. Mol Endocrinol. 1995;9:1346–1355. doi: 10.1210/mend.9.10.8544843. [DOI] [PubMed] [Google Scholar]
- 31.Miller W L. J Steroid Biochem Mol Biol. 1995;55:607–616. doi: 10.1016/0960-0760(95)00212-x. [DOI] [PubMed] [Google Scholar]
- 32.Payne A H, O’Shaughnessy P J. In: The Leydig Cell. Payne A H, Hardy M P, Russell L D, editors. Vienna, IL: Cache River Press; 1996. pp. 259–282. [Google Scholar]
- 33.Parker K L, Schimmer B P. Trends Endocrinol Metab. 1996;7:203–207. doi: 10.1016/1043-2760(96)00105-1. [DOI] [PubMed] [Google Scholar]
- 34.Boujrad N, Ogwuegbu S O, Garnier M, Lee C H, Martin B M, Papadopoulos V. Science. 1995;268:1609–1612. doi: 10.1126/science.7777858. [DOI] [PubMed] [Google Scholar]
- 35.Segaloff D L, Ascoli M. Endocr Rev. 1993;14:324–347. doi: 10.1210/edrv-14-3-324. [DOI] [PubMed] [Google Scholar]
- 36.Rey R A, Campo S M, Bedecárrès P, Nagle C A, Chemes H E. J Clin Endocrinol Metab. 1993;76:1325–1331. doi: 10.1210/jcem.76.5.8496325. [DOI] [PubMed] [Google Scholar]
- 37.Saez J M. Endocr Rev. 1994;15:574–626. doi: 10.1210/edrv-15-5-574. [DOI] [PubMed] [Google Scholar]
- 38.Matzuk M M, Finegold M J, Su J G J, Hsueh A J W, Bradley A. Nature (London) 1992;360:313–319. doi: 10.1038/360313a0. [DOI] [PubMed] [Google Scholar]
- 39.Matzuk M M, Finegold M J, Mishina Y, Bradley A, Behringer R R. Mol Endocrinol. 1995;9:1337–1345. doi: 10.1210/mend.9.10.8544842. [DOI] [PubMed] [Google Scholar]
- 40.Mendis-Handagama S M L C, Gelber S J. Tissue Cell. 1995;27:689–699. doi: 10.1016/s0040-8166(05)80024-2. [DOI] [PubMed] [Google Scholar]
- 41.Teerds K J, de Rooijs D G, Rommerts F F G, Wensing C J G. J Endocrinol. 1990;126:229–236. doi: 10.1677/joe.0.1260229. [DOI] [PubMed] [Google Scholar]
- 42.Murono E P. Acta Endocrinol. 1989;121:477–483. doi: 10.1530/acta.0.1210477. [DOI] [PubMed] [Google Scholar]
- 43.di Clemente N, Goxe B, Remy J J, Cate R L, Josso N, Vigier B, Salesse R. Endocrine. 1994;2:553–558. [Google Scholar]
- 44.Huhtaniemi I P, Katikineni M, Chan V, Catt K J. Endocrinology. 1981;108:58–65. doi: 10.1210/endo-108-1-58. [DOI] [PubMed] [Google Scholar]
- 45.Huhtaniemi I T, Nozu K, Warren D W, Dufau M L, Catt K J. Endocrinology. 1982;111:1721–1727. doi: 10.1210/endo-111-5-1711. [DOI] [PubMed] [Google Scholar]
- 46.Zwain I H, Cheng C Y. Mol Cell Endocrinol. 1994;104:213–227. doi: 10.1016/0303-7207(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 47.Moore A, Krummen L A, Mather J P. Mol Cell Endocrinol. 1994;100:81–86. doi: 10.1016/0303-7207(94)90283-6. [DOI] [PubMed] [Google Scholar]
- 48.Ikeda Y, Shen W H, Ingraham H A, Parker K L. Mol Endocrinol. 1994;8:654–662. doi: 10.1210/mend.8.5.8058073. [DOI] [PubMed] [Google Scholar]
- 49.Jost A, Vigier B, Prépin J. J Reprod Fertil. 1972;29:349–379. doi: 10.1530/jrf.0.0290349. [DOI] [PubMed] [Google Scholar]
- 50.Kim J H, Seibel M M, MacLaughlin D T, Donahoe P K, Ransil B J, Hametz P A, Richards C J. J Clin Endocrinol Metab. 1992;75:911–917. doi: 10.1210/jcem.75.3.1517385. [DOI] [PubMed] [Google Scholar]
- 51.Josso N. Endocr Rev. 1986;7:421–433. doi: 10.1210/edrv-7-4-421. [DOI] [PubMed] [Google Scholar]
- 52.Greco T L, Payne A H. Endocrinology. 1994;135:262–268. doi: 10.1210/endo.135.1.8013361. [DOI] [PubMed] [Google Scholar]
- 53.Clark B J, Wells J, King S R, Stocco D. J Biol Chem. 1994;269:28314–28322. [PubMed] [Google Scholar]
- 54.Tsai-Morris C H, Buczko E, Wang W, Xie X Z, Dufau M L. J Biol Chem. 1991;266:11355–11359. [PubMed] [Google Scholar]
- 55.Sprengel R, Braun T, Nikolics K, Segaloff D L, Seeburg P. Mol Endocrinol. 1990;4:525–530. doi: 10.1210/mend-4-4-525. [DOI] [PubMed] [Google Scholar]
- 56.Nudel U, Zakut R, Shani M, Neuman S, Levy Z, Yaffe D. Nucleic Acids Res. 1983;11:1759–1771. doi: 10.1093/nar/11.6.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Connaughton J F, Rairkar A, Lorckard R E, Kumar A. Nucleic Acids Res. 1984;12:4731–4745. doi: 10.1093/nar/12.11.4731. [DOI] [PMC free article] [PubMed] [Google Scholar]