Abstract
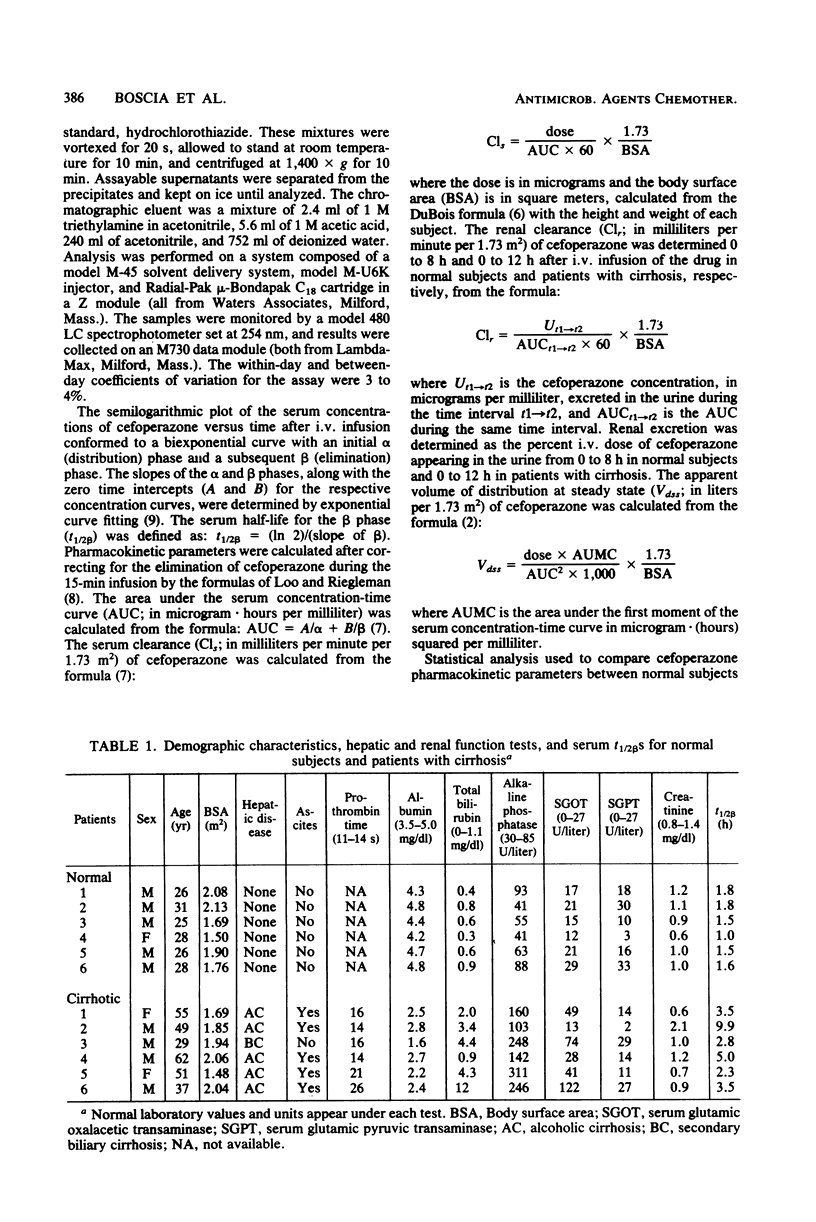
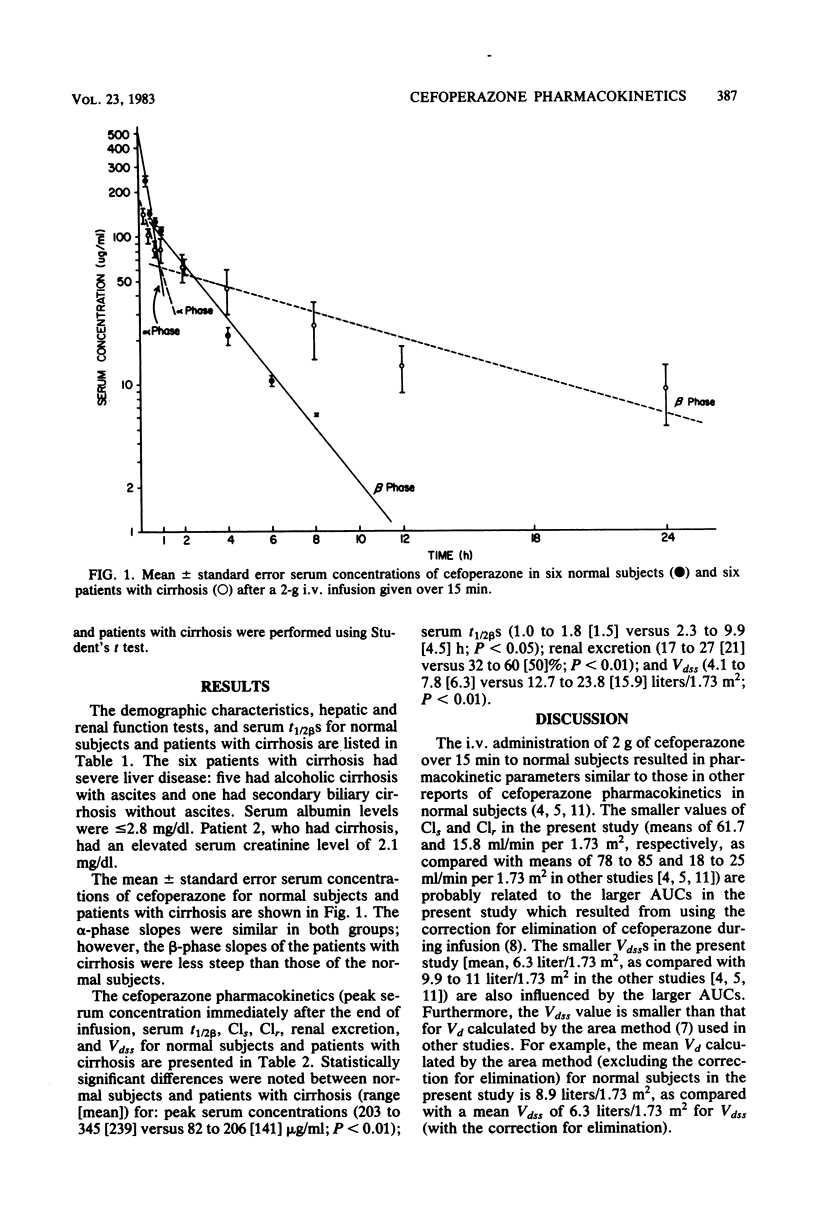
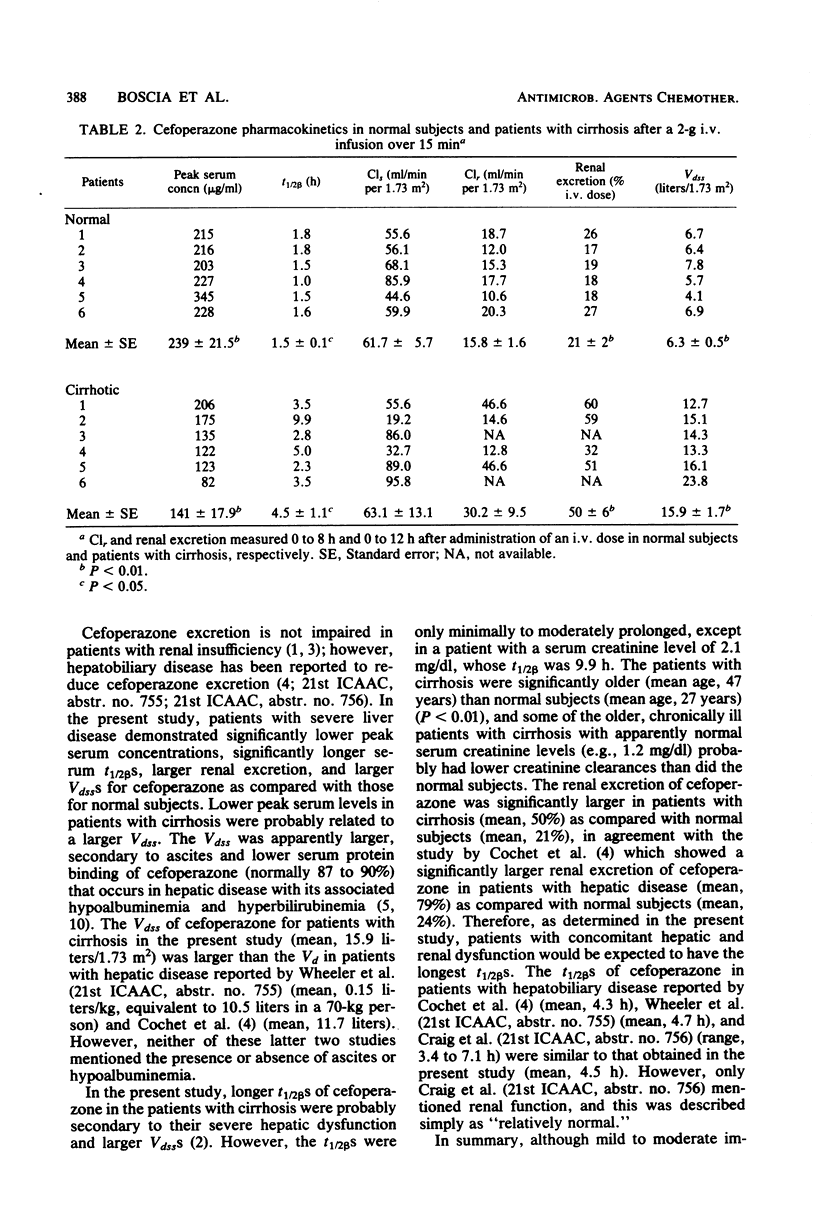
The pharmacokinetics of cefoperazone were studied and compared in six normal subjects and six patients with severe liver disease. All subjects received a 2-g intravenous infusion of cefoperazone over 15 min. Significantly different results were noted between normal subjects and patients with cirrhosis (range [mean]) for the following: peak serum concentrations (203 to 345 [239] versus 82 to 206 [141] micrograms/ml; P less than 0.01); serum beta half-lives (1.0 to 1.8 [1.5] versus 2.3 to 9.9 [4.5] h; P less than 0.05); renal excretion (17 to 27 [21] versus 32 to 60 [50]%; P less than 0.01); and apparent volumes of distribution at steady state (4.1 to 7.8 [6.3] versus 12.7 to 23.8 [15.9] liters/1.73 m2; P less than 0.01). Lower peak serum levels in the patients with cirrhosis were probably related to an increased apparent volume of distribution secondary to ascites and to decreased serum protein binding of cefoperazone. Longer beta half-lives in the patients with cirrhosis were probably secondary to both decreased hepatic excretion caused by severe liver disease and to increased apparent volume of distribution. However, the longest beta half-life among the patients with cirrhosis was in a subject with a serum creatinine level of 2.1 mg/dl. We conclude that, although mild to moderate impairment of cefoperazone excretion occurs in patients with hepatic disease, adjustment of dosage may be necessary only with concomitant renal insufficiency.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailey R. R., Peddie B., Blake E. Serum and urine concentrations of cefoperazone in severe chronic renal failure. Drugs. 1981;22 (Suppl 1):46–51. doi: 10.2165/00003495-198100221-00011. [DOI] [PubMed] [Google Scholar]
- Bolton W. K., Scheld W. M., Spyker D. A., Sande M. A. Pharmacokinetics of cefoperazone in normal volunteers and subjects with renal insufficiency. Antimicrob Agents Chemother. 1981 May;19(5):821–825. doi: 10.1128/aac.19.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig W. A. Single-dose pharmacokinetics of cefoperazone following intravenous administration. Clin Ther. 1980;3(Spec Issue):46–49. [PubMed] [Google Scholar]
- Loo J. C., Riegelman S. Assessment of pharmacokinetic constants from postinfusion blood curves obtained after I.V. infusion. J Pharm Sci. 1970 Jan;59(1):53–55. doi: 10.1002/jps.2600590107. [DOI] [PubMed] [Google Scholar]
- Shimizu K. Cefoperazone: absorption, excretion, distribution, and metabolism. Clin Ther. 1980;3(Spec Issue):60–79. [PubMed] [Google Scholar]
- Srinivasan S., Francke E. L., Neu H. C. Comparative pharmacokinetics of cefoperazone and cefamandole. Antimicrob Agents Chemother. 1981 Feb;19(2):298–301. doi: 10.1128/aac.19.2.298. [DOI] [PMC free article] [PubMed] [Google Scholar]



